RNA Controls PolyQ Protein Phase Transitions
- PMID: 26474065
- PMCID: PMC5221516
- DOI: 10.1016/j.molcel.2015.09.017
RNA Controls PolyQ Protein Phase Transitions
Abstract
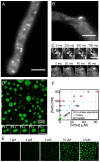
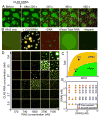
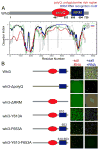
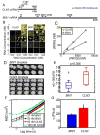
Compartmentalization in cells is central to the spatial and temporal control of biochemistry. In addition to membrane-bound organelles, membrane-less compartments form partitions in cells. Increasing evidence suggests that these compartments assemble through liquid-liquid phase separation. However, the spatiotemporal control of their assembly, and how they maintain distinct functional and physical identities, is poorly understood. We have previously shown an RNA-binding protein with a polyQ-expansion called Whi3 is essential for the spatial patterning of cyclin and formin transcripts in cytosol. Here, we show that specific mRNAs that are known physiological targets of Whi3 drive phase separation. mRNA can alter the viscosity of droplets, their propensity to fuse, and the exchange rates of components with bulk solution. Different mRNAs impart distinct biophysical properties of droplets, indicating mRNA can bring individuality to assemblies. Our findings suggest that mRNAs can encode not only genetic information but also the biophysical properties of phase-separated compartments.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. - PubMed
-
- Caudron F, Barral Y. A super-assembly of Whi3 encodes memory of deceptive encounters by single cells during yeast courtship. Cell. 2013;155:1244–1257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

