Acidosis-Induced Dysfunction of Cortical GABAergic Neurons through Astrocyte-Related Excitotoxicity
- PMID: 26474076
- PMCID: PMC4608795
- DOI: 10.1371/journal.pone.0140324
Acidosis-Induced Dysfunction of Cortical GABAergic Neurons through Astrocyte-Related Excitotoxicity
Abstract
Background: Acidosis impairs cognitions and behaviors presumably by acidification-induced changes in neuronal metabolism. Cortical GABAergic neurons are vulnerable to pathological factors and their injury leads to brain dysfunction. How acidosis induces GABAergic neuron injury remains elusive. As the glia cells and neurons interact each other, we intend to examine the role of the astrocytes in acidosis-induced GABAergic neuron injury.
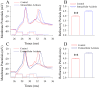
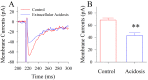
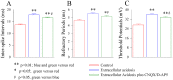
Results: Experiments were done at GABAergic cells and astrocytes in mouse cortical slices. To identify astrocytic involvement in acidosis-induced impairment, we induced the acidification in single GABAergic neuron by infusing proton intracellularly or in both neurons and astrocytes by using proton extracellularly. Compared the effects of intracellular acidification and extracellular acidification on GABAergic neurons, we found that their active intrinsic properties and synaptic outputs appeared more severely impaired in extracellular acidosis than intracellular acidosis. Meanwhile, extracellular acidosis deteriorated glutamate transporter currents on the astrocytes and upregulated excitatory synaptic transmission on the GABAergic neurons. Moreover, the antagonists of glutamate NMDA-/AMPA-receptors partially reverse extracellular acidosis-induced injury in the GABAergic neurons.
Conclusion: Our studies suggest that acidosis leads to the dysfunction of cortical GABAergic neurons by astrocyte-mediated excitotoxicity, in addition to their metabolic changes as indicated previously.
Conflict of interest statement
Figures






Similar articles
-
Cortical GABAergic neurons are more severely impaired by alkalosis than acidosis.BMC Neurol. 2013 Dec 5;13:192. doi: 10.1186/1471-2377-13-192. BMC Neurol. 2013. PMID: 24314112 Free PMC article.
-
More sensitivity of cortical GABAergic neurons than glutamatergic neurons in response to acidosis.Neuroreport. 2016 May 25;27(8):610-6. doi: 10.1097/WNR.0000000000000585. Neuroreport. 2016. PMID: 27116702
-
A sequential impairment of cortical astrocytes and GABAergic neurons during ischemia is improved by mGluR₁,₅ activation.Neurol Sci. 2013 Jul;34(7):1189-95. doi: 10.1007/s10072-012-1220-9. Epub 2012 Oct 31. Neurol Sci. 2013. PMID: 23109136
-
An impairment of cortical GABAergic neurons is involved in alkalosis-induced brain dysfunctions.Biochem Biophys Res Commun. 2012 Mar 23;419(4):627-31. doi: 10.1016/j.bbrc.2012.02.061. Epub 2012 Feb 17. Biochem Biophys Res Commun. 2012. PMID: 22369942
-
mGluR1,5 activation protects cortical astrocytes and GABAergic neurons from ischemia-induced impairment.Neurosci Res. 2013 Feb;75(2):160-6. doi: 10.1016/j.neures.2012.12.002. Epub 2012 Dec 29. Neurosci Res. 2013. PMID: 23280324
Cited by
-
Acidosis, cognitive dysfunction and motor impairments in patients with kidney disease.Nephrol Dial Transplant. 2021 Dec 28;37(Suppl 2):ii4-ii12. doi: 10.1093/ndt/gfab216. Nephrol Dial Transplant. 2021. PMID: 34718761 Free PMC article. Review.
-
Decreased Brain pH as a Shared Endophenotype of Psychiatric Disorders.Neuropsychopharmacology. 2018 Feb;43(3):459-468. doi: 10.1038/npp.2017.167. Epub 2017 Aug 4. Neuropsychopharmacology. 2018. PMID: 28776581 Free PMC article.
-
[Acid-sensing ion channels differentially affect ictal-like and non-ictal-like epileptic activities of mouse hippocampal pyramidal neurons in acidotic extracellular pH].Nan Fang Yi Ke Da Xue Xue Bao. 2020 Jul 30;40(7):972-980. doi: 10.12122/j.issn.1673-4254.2020.07.09. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32895149 Free PMC article. Chinese.
-
Adolescent Δ9-Tetrahydrocannabinol Exposure and Astrocyte-Specific Genetic Vulnerability Converge on Nuclear Factor-κB-Cyclooxygenase-2 Signaling to Impair Memory in Adulthood.Biol Psychiatry. 2019 Jun 1;85(11):891-903. doi: 10.1016/j.biopsych.2018.07.024. Epub 2018 Aug 16. Biol Psychiatry. 2019. PMID: 30219209 Free PMC article.
-
GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression.Oncotarget. 2017 May 30;8(22):35933-35945. doi: 10.18632/oncotarget.16411. Oncotarget. 2017. PMID: 28415589 Free PMC article.
References
-
- DuBose TD. Acidosis and Alkalosis Fauci AS, editor. New York: McGraw-Hill; 1997.
-
- Okuda T, Kurokawa K, Papadakis MA. Fluid and Electrolyte Disorders In: Tierney LM, McPhee SJ, Papadakis MA, editors. Current Medical Diagnosis & Treatment. 37th ed. Stamford: Appleton & Lange; 1998. p. 824–49.
-
- Nowak TJ, Handford AG. Fluid and Electrolyte Imbalances In: Nowak TJ, Handford AG, editors. Pathophysiology. third ed. Boston: Martin J. Lange; 2004. p. 422–45.
-
- Tizianello A, De Ferrari G, Gurreri G, Acquarone N. Effects of metabolic alkalosis, metabolic acidosis and uraemia on whole-body intracellular pH in man. Clin Sci Mol Med. 1977;52(2):125–35. Epub 1977/02/01. . - PubMed
-
- Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14(8):770–6. Epub 1985/08/01. doi: S0196-0644(85)80055-X [pii]. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

