Clinical efficacy, radiographic progression, and safety through 156 weeks of therapy with subcutaneous golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis despite prior methotrexate therapy: final results of the randomized GO-FORTH trial
- PMID: 26474192
- PMCID: PMC4924564
- DOI: 10.3109/14397595.2015.1109762
Clinical efficacy, radiographic progression, and safety through 156 weeks of therapy with subcutaneous golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis despite prior methotrexate therapy: final results of the randomized GO-FORTH trial
Abstract
Objective: To evaluate the safety and efficacy of golimumab + methotrexate (MTX) in Japanese patients with active rheumatoid arthritis (RA).
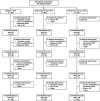
Methods: Japanese patients with active RA despite MTX were randomized to placebo + MTX (Group 1, n = 88), golimumab 50 mg + MTX (Group 2, n = 86), or golimumab 100 mg + MTX (Group 3, n = 87). Patients with <20% improvement in swollen/tender joint counts entered early escape at week 16. At week 24, all remaining placebo patients crossed over to golimumab 50 mg. Efficacy assessments included ACR20, DAS28-ESR, and HAQ-DI. Radiographic progression was assessed with the van der Heijde-modified Sharp (vdH-S) score.
Results: ACR20 response rates in Group 1, Group 2, and Group 3 were 67.9, 86.1, and 82.4%, respectively, at week 52 and were maintained through week 104 (87.1, 94.0, and 88.7%) and week 156 (97.1, 94.1, and 89.5%). Proportions of patients with good/moderate DAS28-ESR response or clinically meaningful improvement in HAQ-DI were also maintained through week 156. The majority of patients did not experience radiographic progression through week 156. Among 257 golimumab-treated patients, 251 (97.7%) had ≥1 AE; 54 (21.0%) had ≥1 serious AE through week 156. Infections were the most common type of AE.
Conclusions: Response to golimumab + MTX was maintained over 3 years in Japanese patients with active RA despite MTX. Safety results were consistent with the known safety profile of golimumab.
Keywords: Anti-tumor necrosis factor; Asian; Golimumab; Rheumatoid arthritis.
Figures


References
-
- Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24:33–40. - PubMed
-
- Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–17. - PubMed
-
- Magnano MD, Genovese MC. Management of co-morbidities and general medical conditions in patients with rheumatoid arthritis. Curr Rheumatol Rep. 2005;7:407–15. - PubMed
-
- Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907–27. - PubMed
-
- Baumgartner SW, Fleischmann RM, Moreland LW, Schiff MH, Markenson J, Whitmore JB. Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol. 2004;31:1532–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

