ApoE Receptor 2 Mediation of Trophoblast Dysfunction and Pregnancy Complications Induced by Antiphospholipid Antibodies in Mice
- PMID: 26474194
- PMCID: PMC4767551
- DOI: 10.1002/art.39453
ApoE Receptor 2 Mediation of Trophoblast Dysfunction and Pregnancy Complications Induced by Antiphospholipid Antibodies in Mice
Abstract
Objective: Pregnancies in women with the antiphospholipid syndrome (APS) are frequently complicated by fetal loss and intrauterine growth restriction (IUGR). How circulating antiphospholipid antibodies (aPL) cause pregnancy complications in APS is poorly understood. We sought to determine whether the low-density lipoprotein receptor family member apolipoprotein E receptor 2 (ApoER2) mediates trophoblast dysfunction and pregnancy complications induced by aPL.
Methods: Placental and trophoblast ApoER2 expression was evaluated by immunohistochemistry and immunoblotting. Normal human IgG and aPL were purified from healthy individuals and APS patients, respectively. The role of ApoER2 in aPL-induced changes in trophoblast proliferation and migration and in kinase activation was assessed using RNA interference in HTR-8/SVneo cells. The participation of ApoER2 in aPL-induced pregnancy loss and IUGR was evaluated in pregnant ApoER2(+/+) and ApoER2(-/-) mice injected with aPL or normal human IgG.
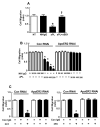
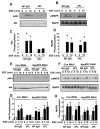
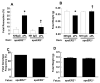
Results: We found that ApoER2 is abundant in human and mouse placental trophoblasts and in multiple trophoblast-derived cell lines, including HTR-8/SVneo cells. ApoER2 and its interaction with the cell surface protein β2 -glycoprotein I were required for aPL-induced inhibition of cultured trophoblast proliferation and migration. In parallel, aPL antagonism of Akt kinase activation by epidermal growth factor in trophoblasts was mediated by ApoER2. Furthermore, in a murine passive-transfer model of pregnancy complications of APS, ApoER2(-/-) mice were protected from both aPL-induced fetal loss and aPL-induced IUGR.
Conclusion: ApoER2 plays a major role in the attenuation of trophoblast function by aPL, and the receptor mediates aPL-induced pregnancy complications in vivo in mice. ApoER2-directed interventions can now potentially be developed to combat the pregnancy complications associated with APS.
© 2015, American College of Rheumatology.
Figures



Comment in
-
Connective tissue diseases: ApoER2 linked to pregnancy complications in APS.Nat Rev Rheumatol. 2015 Dec;11(12):684. doi: 10.1038/nrrheum.2015.150. Epub 2015 Nov 3. Nat Rev Rheumatol. 2015. PMID: 26526645 No abstract available.
References
-
- Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7(6):330–9. - PubMed
-
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–509. - PubMed
-
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. - PubMed
-
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346(10):752–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

