Identification of drugs as single agents or in combination to prevent carcinoma dissemination in a microfluidic 3D environment
- PMID: 26474384
- PMCID: PMC4742198
- DOI: 10.18632/oncotarget.5464
Identification of drugs as single agents or in combination to prevent carcinoma dissemination in a microfluidic 3D environment
Abstract
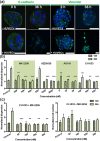
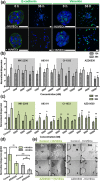
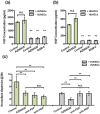
Experiments were performed in a modified microfluidic platform recapitulating part of the in vivo tumor microenvironment by co-culturing carcinoma cell aggregates embedded in a three-dimensional (3D) collagen scaffold with human umbilical vein endothelial cells (HUVECs). HUVECs were seeded in one channel of the device to initiate vessel-like structures in vitro prior to introducing the aggregates. The lung adenocarcinoma cell line A549 and the bladder carcinoma cell line T24 were tested. Dose-response assays of four drugs known to interfere with Epithelial Mesenchymal Transition (EMT) signaling pathways were quantified using relative dispersion as a metric of EMT progression. The presence of HUVECs in one channel induces cell dispersal in A549 which then can be inhibited by each of the four drugs. Complete inhibition of T24 aggregate dispersal, however, is not achieved with any single agent, although partial inhibition was observed with 10 μM of the Src inhibitor, AZD-0530. Almost complete inhibition of T24 dispersal in monoculture was achieved only when the four drugs were added in combination, each at 10 μM concentration. Coculture of T24 with HUVECs forfeits the almost-complete inhibition. The enhanced dispersal observed in the presence of HUVECs is a consequence of secretion of growth factors, including HGF and FGF-2, by endothelial cells. This 3D microfluidic co-culture platform provides an in vivo-like surrogate for anti-invasive and anti-metastatic drug screening. It will be particularly useful for defining combination therapies for aggressive tumors such as invasive bladder carcinoma.
Keywords: bladder cancer; drug screening; epithelial-mesenchymal transition; microfluidics; synergistic effect.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures



References
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews Cancer. 2002;2:442–454. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

