The Features of Copper Metabolism in the Rat Liver during Development
- PMID: 26474410
- PMCID: PMC4608700
- DOI: 10.1371/journal.pone.0140797
The Features of Copper Metabolism in the Rat Liver during Development
Abstract
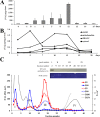
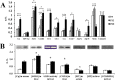
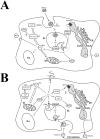
Strong interest in copper homeostasis is due to the fact that copper is simultaneously a catalytic co-factor of the vital enzymes, a participant in signaling, and a toxic agent provoking oxidative stress. In mammals, during development copper metabolism is conformed to two types. In embryonic type copper metabolism (ETCM), newborns accumulate copper to high level in the liver because its excretion via bile is blocked; and serum copper concentration is low because ceruloplasmin (the main copper-containing protein of plasma) gene expression is repressed. In the late weaning, the ETCM switches to the adult type copper metabolism (ATCM), which is manifested by the unlocking of copper excretion and the induction of ceruloplasmin gene activity. The considerable progress has been made in the understanding of the molecular basis of copper metabolic turnover in the ATCM, but many aspects of the copper homeostasis in the ETCM remain unclear. The aim of this study was to investigate the copper metabolism during transition from the ETCM (up to 12-days-old) to the ATCM in the rats. It was shown that in the liver, copper was accumulated in the nuclei during the first 5 days of life, and then it was re-located to the mitochondria. In parallel with the mitochondria, copper bulk bound with cytosolic metallothionein was increased. All compartments of the liver cells rapidly lost most of their copper on the 13th day of life. In newborns, serum copper concentration was low, and its major fraction was associated with holo-Cp, however, a small portion of copper was bound to extracellular metallothionein and a substance that was slowly eluted during gel-filtration. In adults, serum copper concentration increased by about a factor of 3, while metallothionein-bound copper level decreased by a factor of 2. During development, the expression level of Cp, Sod1, Cox4i1, Atp7b, Ctr1, Ctr2, Cox17, and Ccs genes was significantly increased, and metallothionein was decreased. Atp7a gene's activity was fully repressed. The copper routes in newborns are discussed.
Conflict of interest statement
Figures

References
-
- Kozlowski H, Kozlkowska P, Watly J, Krzywoszynska K, Potocki S. General Aspects of Metal Toxicity. Curr Med Chem. 2014; 21: 3721–3740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

