Differentiation and transdifferentiation potentials of cancer stem cells
- PMID: 26474460
- PMCID: PMC4741845
- DOI: 10.18632/oncotarget.6098
Differentiation and transdifferentiation potentials of cancer stem cells
Abstract
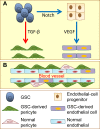
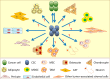
Tumor cells actively contribute to constructing their own microenvironment during tumorigenesis and tumor progression. The tumor microenvironment contains multiple types of stromal cells that work together with the extracellular matrix and local and systemic factors to coordinately contribute to tumor initiation and progression. Tumor cells and their stromal compartments acquire many genetic and/or epigenetic alternations to facilitate tumor growth and metastasis. The cancer stem cell (CSC) concept has been widely applied to interpreting tumor initiation, growth, metastasis, dormancy and relapse. CSCs have differentiation abilities to generate the original lineage cells that are similar to their normal stem cell counterparts. Interestingly, recent evidence demonstrates that CSCs also have the potential to transdifferentiate into vascular endothelial cells and pericytes, indicating that CSCs can transdifferentiate into other lineage cells for promoting tumor growth and metastasis in some tissue contexts instead of only recruiting stromal cells from local or distant tissues. Although the transdifferentiation of CSCs into tumor stromal cells provides a new dimension that explains tumor heterogeneity, many aspects of CSC transdifferentiation remain elusive. In this review, we summarize the multi-lineage differentiation and transdifferentiation potentials of CSCs as well as discuss their potential contributions to tumor heterogeneity and tumor microenvironment in tumor progression.
Keywords: cancer stem cell; differentiation; stromal cells; transdifferentiation; tumor microenvironment.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures

Similar articles
-
Revisiting Cancer Stem Cells as the Origin of Cancer-Associated Cells in the Tumor Microenvironment: A Hypothetical View from the Potential of iPSCs.Cancers (Basel). 2020 Apr 4;12(4):879. doi: 10.3390/cancers12040879. Cancers (Basel). 2020. PMID: 32260363 Free PMC article. Review.
-
Tumor microenvironment for cancer stem cells.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt B):197-205. doi: 10.1016/j.addr.2015.08.005. Epub 2015 Sep 8. Adv Drug Deliv Rev. 2016. PMID: 26362921 Review.
-
Targeting CSCs within the tumor microenvironment for cancer therapy: a potential role of mesenchymal stem cells.Expert Opin Ther Targets. 2012 Oct;16(10):1041-54. doi: 10.1517/14728222.2012.714774. Epub 2012 Aug 9. Expert Opin Ther Targets. 2012. PMID: 22877147 Review.
-
Cancer stem cells, lymphangiogenesis, and lymphatic metastasis.Cancer Lett. 2015 Feb 28;357(2):438-47. doi: 10.1016/j.canlet.2014.12.013. Epub 2014 Dec 10. Cancer Lett. 2015. PMID: 25497008 Review.
-
Breast-cancer stem cells-beyond semantics.Lancet Oncol. 2012 Jan;13(1):e43-8. doi: 10.1016/S1470-2045(11)70191-7. Lancet Oncol. 2012. PMID: 22225725 Review.
Cited by
-
The Great Escape: The Power of Cancer Stem Cells to Evade Programmed Cell Death.Cancers (Basel). 2021 Jan 17;13(2):328. doi: 10.3390/cancers13020328. Cancers (Basel). 2021. PMID: 33477367 Free PMC article. Review.
-
miR-326 inhibits the cell proliferation and cancer stem cell-like property of cervical cancer in vitro and oncogenesis in vivo via targeting TCF4.Ann Transl Med. 2020 Dec;8(24):1638. doi: 10.21037/atm-20-6830. Ann Transl Med. 2020. PMID: 33490150 Free PMC article.
-
Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies.Cancers (Basel). 2025 Jan 24;17(3):382. doi: 10.3390/cancers17030382. Cancers (Basel). 2025. PMID: 39941751 Free PMC article. Review.
-
Expression of DNAJB1-PRKACA oncogene suppresses the differentiation potential of liver progenitor organoids towards a hepatocyte lineage.Sci Rep. 2025 Jul 16;15(1):25796. doi: 10.1038/s41598-025-11028-4. Sci Rep. 2025. PMID: 40670531 Free PMC article.
-
Signaling pathways in the regulation of cancer stem cells and associated targeted therapy.MedComm (2020). 2022 Oct 5;3(4):e176. doi: 10.1002/mco2.176. eCollection 2022 Dec. MedComm (2020). 2022. PMID: 36226253 Free PMC article. Review.
References
-
- Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. - PubMed
-
- Wang Z, Ouyang G. Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell. 2012;10:111–112. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

