Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response
- PMID: 26474549
- PMCID: PMC4609167
- DOI: 10.1186/s12885-015-1701-3
Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response
Abstract
Background: This retrospective study aims to investigate the activity of retreatment with anti-EGFR-based therapies in order to explore the concept of clonal evolution by evaluating the impact of prior activity and intervening time interval.
Methods: Eighty-nine KRAS exon 2-wild-type metastatic colorectal patients were retreated on phase I/II clinical trials containing anti-EGFR therapies after progressing on prior cetuximab or panitumumab. Response on prior anti-EGFR therapy was defined retrospectively per physician-records as response or stable disease ≥6 months. Multivariable statistical methods included a multiple logistic regression model for response, and Cox proportional hazards model for progression-free survival.
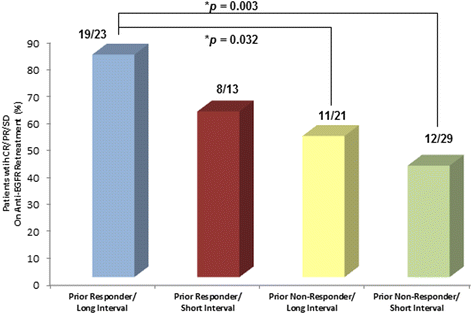
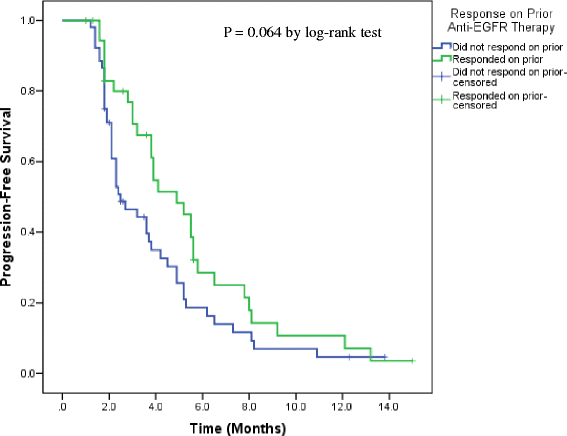
Results: Retreatment anti-EGFR agents were cetuximab (n = 76) or cetuximab plus erlotinib (n = 13). The median interval time between prior and retreatment regimens was 4.57 months (range: 0.46-58.7). Patients who responded to the prior cetuximab or panitumumab were more likely to obtain clinical benefit to the retreatment compared to the non-responders in both univariate (p = 0.007) and multivariate analyses (OR: 3.38, 95 % CI: 1.27, 9.31, p = 0.019). The clinical benefit rate on retreatment also showed a marginally significant association with interval time between the two anti-EGFR based therapies (p = 0.053). Median progression-free survival on retreatment was increased in prior responders (4.9 months, 95 % CI: 3.6, 6.2) compared to prior non-responders (2.5 months, 95 % CI, 1.58, 3.42) in univariate (p = 0.064) and multivariate analysis (HR: 0.70, 95 % CI: 0.43-1.15, p = 0.156).
Conclusion: Our data lends support to the concept of clonal evolution, though the clinical impact appears less robust than previously reported. Further work to determine which patients benefit from retreatment post progression is needed.
Figures
Similar articles
-
Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab.Oncologist. 2012;17(1):14. doi: 10.1634/theoncologist.2011-0452. Epub 2011 Dec 30. Oncologist. 2012. PMID: 22210091 Free PMC article. Clinical Trial.
-
Cetuximab plus irinotecan versus panitumumab in patients with refractory metastatic colorectal cancer in Ontario, Canada.Int J Cancer. 2017 May 1;140(9):2162-2167. doi: 10.1002/ijc.30637. Int J Cancer. 2017. PMID: 28220486
-
Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the phase II DUX study in chemotherapy-refractory, advanced colorectal cancer.J Clin Oncol. 2012 May 1;30(13):1505-12. doi: 10.1200/JCO.2011.38.6599. Epub 2012 Mar 12. J Clin Oncol. 2012. PMID: 22412142 Clinical Trial.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
-
Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis.Eur J Cancer. 2015 Mar;51(5):587-94. doi: 10.1016/j.ejca.2015.01.054. Epub 2015 Feb 9. Eur J Cancer. 2015. PMID: 25673558 Review.
Cited by
-
Biomarker-Guided Anti-Egfr Rechallenge Therapy in Metastatic Colorectal Cancer.Cancers (Basel). 2021 Apr 17;13(8):1941. doi: 10.3390/cancers13081941. Cancers (Basel). 2021. PMID: 33920531 Free PMC article. Review.
-
Rechallenge and maintenance therapy using cetuximab and chemotherapy administered to a patient with metastatic colorectal cancer.BMC Cancer. 2017 Feb 14;17(1):132. doi: 10.1186/s12885-017-3133-8. BMC Cancer. 2017. PMID: 28196490 Free PMC article.
-
REMARRY and PURSUIT trials: liquid biopsy-guided rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab plus irinotecan for patients with plasma RAS wild-type metastatic colorectal cancer.BMC Cancer. 2021 Jun 7;21(1):674. doi: 10.1186/s12885-021-08395-2. BMC Cancer. 2021. PMID: 34098908 Free PMC article.
-
Liquid Biopsy-Driven Cetuximab Rechallenge Strategy in Molecularly Selected Metastatic Colorectal Cancer Patients.Front Oncol. 2022 Apr 21;12:852583. doi: 10.3389/fonc.2022.852583. eCollection 2022. Front Oncol. 2022. PMID: 35530345 Free PMC article.
-
Prognostic value of carbohydrate antigen125 and carcino embryonic antigen expression in patients with colorectal carcinoma and its guiding significance for chemotherapy.Medicine (Baltimore). 2020 Apr;99(14):e19420. doi: 10.1097/MD.0000000000019420. Medicine (Baltimore). 2020. PMID: 32243362 Free PMC article.
References
-
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30(21):2585–2592. doi: 10.1200/JCO.2011.35.6725. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

