Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport
- PMID: 26474562
- PMCID: PMC4609144
- DOI: 10.1186/s12951-015-0131-3
Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport
Abstract
Background: Due to bacterial resistance to antibiotics there is a need for new antimicrobial agents. In this respect nanoparticles can be used as they have expressed antibacterial activity simultaneously being more reactive compared to their bulk material. The action of zinc (II), titanium (IV), copper (II) and (I) oxides thin films with nanostructured surface and silver nanoscale particles on Enterococcus hirae and Escherichia coli growth and membrane activity was studied by using microbiological, potentiometric and spectrophotometric methods.
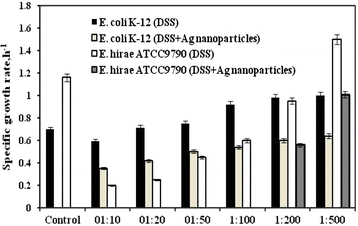
Results: It was revealed that sapphire base plates with deposited ZnO, TiO2, CuO and Cu2O nanoparticles had no effects neither on E. hirae nor E. coli growth both on agar plates and in liquid medium. Concentrated Ag nanoparticles colloid solution markedly affected bacterial growth which was expressed by changing growth properties. E. hirae was able to grow only at <1:200 dilutions of Ag nanoparticles while E. coli grew even at 1:10 dilution. At the same time Ag nanoparticles directly affected membranes, as the FOF1-ATPase activity and H(+)-coupled transport was changed either (E. coli were less susceptible to nanoparticles compared to E. hirae). Ag nanoparticles increased H(+) and K(+) transport even in the presence of N,N'-dicyclohexylcarbodiimide (DCCD), inhibitor of FOF1. The stoichiometry of DCCD-inhibited ion fluxes was disturbed.
Conclusions: These results point out to distinguishing antibacterial effects of Ag nanoparticles on different bacteria; the difference between effects can be explained by peculiarities in bacterial membrane structure and properties. H(+)-K(+)-exchange disturbance by Ag nanoparticles might be involved in antibacterial effects on E. hirae. The role of FOF1 in antibacterial action of Ag nanoparticles was shown using atpD mutant lacked β subunit in F1.
Figures




Similar articles
-
The effects of manganese (II) but not nickel (II) ions on Enterococcus hirae cell growth, redox potential decrease, and proton-coupled membrane transport.Cell Biochem Biophys. 2013;67(3):1301-6. doi: 10.1007/s12013-013-9662-0. Cell Biochem Biophys. 2013. PMID: 23712873
-
The effects of copper (II) ions on Enterococcus hirae cell growth and the proton-translocating FoF1 ATPase activity.Cell Biochem Biophys. 2010 May;57(1):19-26. doi: 10.1007/s12013-010-9078-z. Cell Biochem Biophys. 2010. PMID: 20352375
-
Antibacterial effects of iron oxide (Fe3O4) nanoparticles: distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms.Appl Microbiol Biotechnol. 2019 Mar;103(6):2773-2782. doi: 10.1007/s00253-019-09653-x. Epub 2019 Feb 1. Appl Microbiol Biotechnol. 2019. PMID: 30706116
-
Cu(II), Fe(III) and Mn(II) combinations as environmental stress factors have distinguishing effects on Enterococcus hirae.J Environ Sci (China). 2015 Feb 1;28:95-100. doi: 10.1016/j.jes.2014.06.043. Epub 2014 Dec 3. J Environ Sci (China). 2015. PMID: 25662243
-
Antibacterial activities of transient metals nanoparticles and membranous mechanisms of action.World J Microbiol Biotechnol. 2019 Oct 14;35(10):162. doi: 10.1007/s11274-019-2742-6. World J Microbiol Biotechnol. 2019. PMID: 31612285 Review.
Cited by
-
Enhanced Antibacterial Activity of Silver Nanoparticles Combined with Hydrogen Peroxide Against Multidrug-Resistant Pathogens Isolated from Dairy Farms and Beef Slaughterhouses in Egypt.Infect Drug Resist. 2020 Oct 8;13:3485-3499. doi: 10.2147/IDR.S271261. eCollection 2020. Infect Drug Resist. 2020. PMID: 33116668 Free PMC article.
-
Antibacterial and antibiofilm effects of Pseudomonas aeruginosa derived outer membrane vesicles against Streptococcus mutans.Heliyon. 2023 Nov 26;9(12):e22606. doi: 10.1016/j.heliyon.2023.e22606. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125454 Free PMC article.
-
Antibacterial Efficacy of Silver Nanoparticles on Endometritis Caused by Prevotella melaninogenica and Arcanobacterum pyogenes in Dairy Cattle.Int J Mol Sci. 2018 Apr 16;19(4):1210. doi: 10.3390/ijms19041210. Int J Mol Sci. 2018. PMID: 29659523 Free PMC article.
-
Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy.Int J Mol Sci. 2017 Mar 6;18(3):569. doi: 10.3390/ijms18030569. Int J Mol Sci. 2017. PMID: 28272303 Free PMC article.
-
Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles.Rev Inst Med Trop Sao Paulo. 2018;60:e18. doi: 10.1590/s1678-9946201860018. Epub 2018 Apr 23. Rev Inst Med Trop Sao Paulo. 2018. PMID: 29694600 Free PMC article.
References
-
- Ansari MA, Khan HM, Khan AA, Malik A, Sultan A, Shahid M, et al. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol Med. 2011;3:141–146.
-
- Bhati-Kushwaha H, Malik CP. Assessment of antibacterial and antifungal activities of silver nanoparticles obtained from the Callus (stem and leaf) of Tridax procumbens L. Ind J Biotechnol. 2014;13:114–120.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

