Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin
- PMID: 26474767
- PMCID: PMC4609080
- DOI: 10.1186/s13287-015-0195-x
Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin
Abstract
Introduction: Osteoporosis is a syndrome of excessive skeletal fragility characterized by the loss of mass and deterioration of microarchitecture in bone. Single use of aspirin or adipose-derived stromal cells (ASCs) has been recognized recently to be effective against osteoporosis. The goal of the study was to evaluate the osteogenic effects of the co-administration of aspirin and allogeneic rat adipose-derived stromal cells (rASCs) on ovariectomized (OVX)-induced bone loss in rats. The underlying mechanisms were investigated in vitro and in vivo.
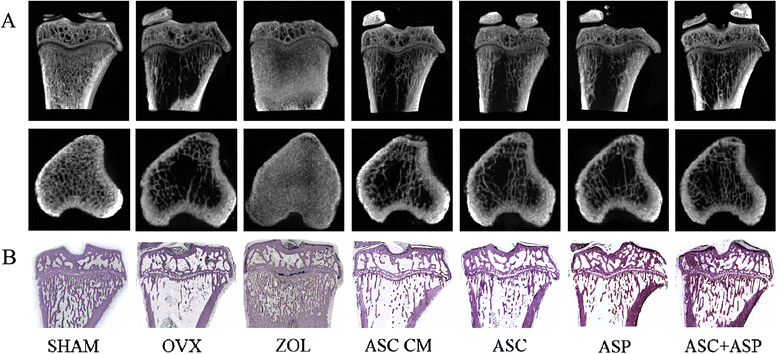
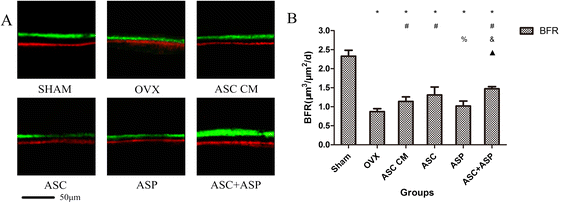
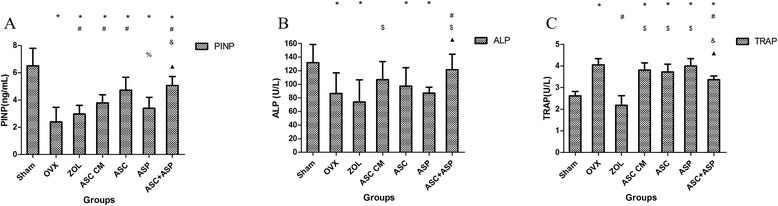
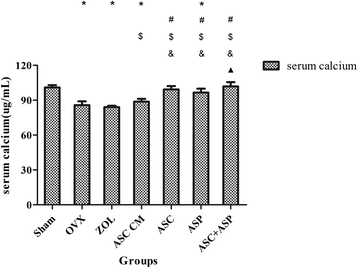
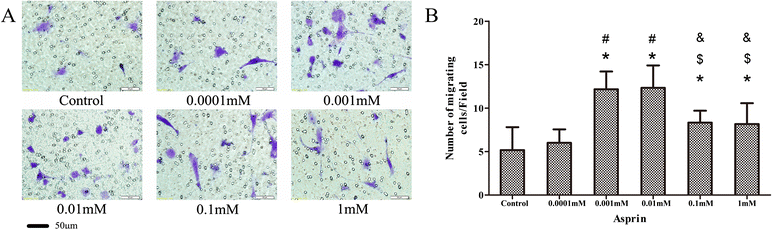
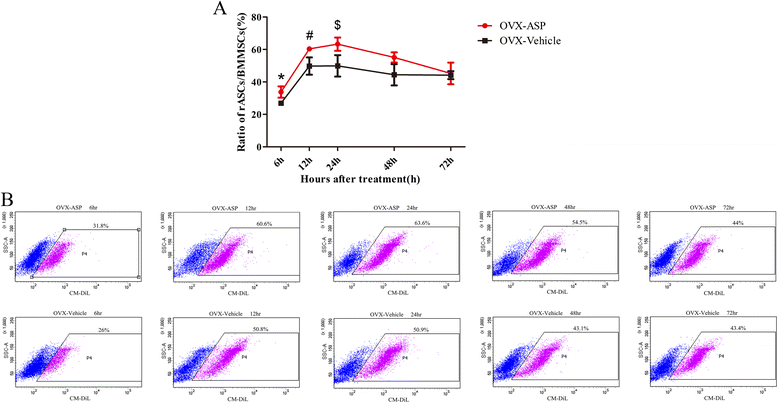
Methods: Firstly, allogeneic rASCs were isolated and cultured, and the conditioned medium (CM) from the maintenance of rASCs was collected. Secondly, the OVX rats were administrated CM, rASCs, aspirin (ASP) or rASCs + ASP, respectively. Twelve weeks later, the anti-inflammatory and osteogenic effects were assessed by micro-CT, undecalcified histological sections, dynamic histomorphometric analyses and serologic assays for biochemical markers. Finally, a Transwell migration assay in vitro and cell-trafficking analyses in vivo were used to explore the effects of aspirin on rASC migration.
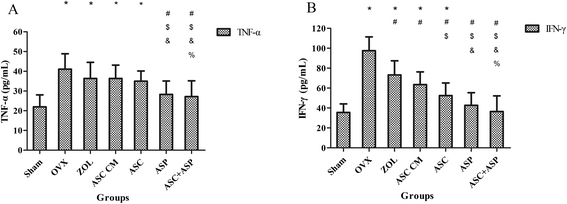
Results: Systemic administration of aspirin and rASCs attenuated OVX-induced bone loss better than single use of aspirin or ASCs (p < 0.05, respectively). Next, we analyzed the underlying mechanisms of the anti-inflammatory and chemotactic abilities of aspirin. Aspirin suppressed serum levels of the pro-inflammatory cytokines on tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and the anti-inflammatory ability was positively associated with bone morphometry. Also, aspirin exhibited excellent chemotactic effects in vitro and accelerated the homing of allogeneic rASCs into bone marrow during early in vivo stages.
Conclusions: Co-administered aspirin and allogeneic ASCs can partially reverse OVX-induced bone loss in rats. This effect appears to be mediated by the anti-inflammatory and chemotactic abilities of aspirin.
Figures
Similar articles
-
Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model.Cytotherapy. 2014 Dec;16(12):1643-55. doi: 10.1016/j.jcyt.2014.07.009. Epub 2014 Sep 16. Cytotherapy. 2014. PMID: 25231892
-
Systemic Administration of Allogeneic Mesenchymal Stem Cells Does Not Halt Osteoporotic Bone Loss in Ovariectomized Rats.PLoS One. 2016 Oct 6;11(10):e0163131. doi: 10.1371/journal.pone.0163131. eCollection 2016. PLoS One. 2016. PMID: 27711227 Free PMC article.
-
The interactions between rat-adipose-derived stromal cells, recombinant human bone morphogenetic protein-2, and beta-tricalcium phosphate play an important role in bone tissue engineering.Tissue Eng Part A. 2010 Sep;16(9):2927-40. doi: 10.1089/ten.TEA.2010.0018. Tissue Eng Part A. 2010. PMID: 20486786
-
Transplantation of Hepatocyte Growth Factor-Modified Dental Pulp Stem Cells Prevents Bone Loss in the Early Phase of Ovariectomy-Induced Osteoporosis.Hum Gene Ther. 2018 Feb;29(2):271-282. doi: 10.1089/hum.2017.091. Epub 2017 Nov 10. Hum Gene Ther. 2018. PMID: 28950723
-
Transplantation of Adipose Tissue-Derived Mesenchymal Stem Cell (ATMSC) Expressing Alpha-1 Antitrypsin Reduces Bone Loss in Ovariectomized Osteoporosis Mice.Hum Gene Ther. 2017 Feb;28(2):179-189. doi: 10.1089/hum.2016.069. Epub 2016 Nov 1. Hum Gene Ther. 2017. PMID: 27802778 Review.
Cited by
-
Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration.Cell Biosci. 2019 Dec 23;9:103. doi: 10.1186/s13578-019-0369-9. eCollection 2019. Cell Biosci. 2019. PMID: 31890152 Free PMC article. Review.
-
Injectable nanohydroxyapatite-chitosan-gelatin micro-scaffolds induce regeneration of knee subchondral bone lesions.Sci Rep. 2017 Dec 1;7(1):16709. doi: 10.1038/s41598-017-17025-6. Sci Rep. 2017. PMID: 29196647 Free PMC article.
-
Administration of raloxifene hydrochloride nanosuspensions partially attenuates bone loss in ovariectomized mice.RSC Adv. 2018 Jun 29;8(42):23748-23756. doi: 10.1039/c8ra02535e. eCollection 2018 Jun 27. RSC Adv. 2018. PMID: 35540259 Free PMC article.
-
Platelet factor 4 induces bone loss by inhibiting the integrin α5-FAK-ERK pathway.Animal Model Exp Med. 2023 Dec;6(6):573-584. doi: 10.1002/ame2.12342. Epub 2023 Aug 11. Animal Model Exp Med. 2023. PMID: 37565509 Free PMC article.
-
Effects of Aspirin on Odontogenesis of Human Dental Pulp Cells and TGF-β1 Liberation from Dentin In Vitro.Int J Dent. 2022 Aug 5;2022:3246811. doi: 10.1155/2022/3246811. eCollection 2022. Int J Dent. 2022. PMID: 36034475 Free PMC article.
References
-
- Mulgund M, Beattie KA, Wong AK, Papaioannou A, Adachi JD. Assessing adherence to teriparatide therapy, causes of nonadherence and effect of adherence on bone mineral density measurements in osteoporotic patients at high risk for fracture. Ther Adv Musculoskelet Dis. 2009;1:5–11. doi: 10.1177/1759720X09339551. - DOI - PMC - PubMed
-
- Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, et al. Evidence-based guidelines for the pharmacological treatment of postmenopausal osteoporosis: a consensus document by the Belgian Bone Club. Osteoporos Int. 2010;21:1657–80. doi: 10.1007/s00198-010-1223-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

