Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice
- PMID: 26474831
- PMCID: PMC4640729
- DOI: 10.1073/pnas.1518540112
Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice
Abstract
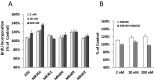
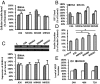
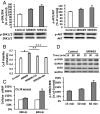
Agonists of growth hormone-releasing hormone (GHRH) have been previously reported to promote growth, function, and engraftment of islet cells following transplantation. Here we evaluated recently synthesized GHRH agonists on the proliferation and biological functions of rat pancreatic β-cell line (INS-1) and islets. In vitro treatment of INS-1 cells with GHRH agonists increased cell proliferation, the expression of cellular insulin, insulin-like growth factor-1 (IGF1), and GHRH receptor, and also stimulated insulin secretion in response to glucose challenge. Exposure of INS-1 cells to GHRH agonists, MR-356 and MR-409, induced activation of ERK and AKT pathways. Agonist MR-409 also significantly increased the levels of cellular cAMP and the phosphorylation of cAMP response element binding protein (CREB) in INS-1 cells. Treatment of rat islets with agonist, MR-409 significantly increased cell proliferation, islet size, and the expression of insulin. In vivo daily s.c. administration of 10 μg MR-409 for 3 wk dramatically reduced the severity of streptozotocin (STZ)-induced diabetes in nonobese diabetic severe combined immunodeficiency (NOD/SCID) mice. The maximal therapeutic benefits with respect to the efficiency of engraftment, ability to reach normoglycemia, gain in body weight, response to high glucose challenge, and induction of higher levels of serum insulin and IGF1 were observed when diabetic mice were transplanted with rat islets preconditioned with GHRH agonist, MR-409, and received additional treatment with MR-409 posttransplantation. This study provides an improved approach to the therapeutic use of GHRH agonists in the treatment of diabetes mellitus.
Keywords: GHRH agonists; INS-1; diabetes; signaling pathways; transplantation.
Conflict of interest statement
Conflict of interest statement: R.C., A.V.S., and N.L.B. are co-inventors on the patent on GHRH agonists, assigned to the University of Miami and the Veterans Affairs. N.L.B. is a member of the board of directors of Biscayne Pharmaceuticals, Inc.
Figures
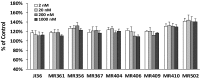



References
-
- Lehmann R, Spinas GA, Moritz W, Weber M. Has time come for new goals in human islet transplantation? Am J Transplant. 2008;8(6):1096–1100. - PubMed
-
- Ludwig B, et al. Islet transplantation at the Dresden diabetes center: Five years’ experience. Horm Metab Res. 2015;47(1):4–8. - PubMed
-
- Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

