Parental origin impairment of synaptic functions and behaviors in cytoplasmic FMRP interacting protein 1 (Cyfip1) deficient mice
- PMID: 26474913
- PMCID: PMC4744651
- DOI: 10.1016/j.brainres.2015.10.015
Parental origin impairment of synaptic functions and behaviors in cytoplasmic FMRP interacting protein 1 (Cyfip1) deficient mice
Abstract
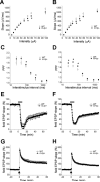
CYFIP1 maps to the interval between proximal breakpoint 1 (BP1) and breakpoint 2 (BP2) of chromosomal 15q11-q13 deletions that are implicated in the Angelman (AS) and Prader-Willi syndrome (PWS). There is only one breakpoint (BP3) at the distal end of deletion. CYFIP1 is deleted in AS patients with the larger class I deletion (BP1 to BP3) and the neurological presentations in these patients are more severe than that of patients with class II (BP2 to BP3) deletion. The haploinsufficiency of CYFIP1 is hypothesized to contribute to more severe clinical presentations in class I AS patients. The expression of CYFIP1 is suggested to be bi-allelic in literature but the possibility of parental origin of expression is not completely excluded. We generated and characterized Cyfip1 mutant mice. Homozygous Cyfip1 mice were early embryonic lethal. However, there was a parental origin specific effect between paternal Cyfip1 deficiency (m+/p-) and maternal deficiency (m-/p+) on both synaptic transmissions and behaviors in hippocampal CA1 synapses despite no evidence supporting the parental origin difference for the expression. Both m-/p+ and m+/p- showed the impaired input-output response and paired-pulse facilitation. While the long term-potentiation and group I mGluR mediated long term depression induced by DHPG was not different between Cyfip1 m-/p+ and m+/p- mice, the initial DHPG induced response was significantly enhanced in m-/p+ but not in m+/p- mice. m+/p- but not m-/p+ mice displayed increased freezing in cued fear conditioning and abnormal transitions in zero-maze test. The impaired synaptic transmission and behaviors in haploinsufficiency of Cyfip1 mice provide the evidence supporting the role of CYFIP1 modifying the clinical presentation of class I AS patients and in human neuropsychiatric disorders.
Keywords: Parental origin.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Cyfip1 Haploinsufficiency Increases Compulsive-Like Behavior and Modulates Palatable Food Intake in Mice: Dependence on Cyfip2 Genetic Background, Parent-of Origin, and Sex.G3 (Bethesda). 2019 Sep 4;9(9):3009-3022. doi: 10.1534/g3.119.400470. G3 (Bethesda). 2019. PMID: 31324746 Free PMC article.
-
Detection of a novel familial deletion of four genes between BP1 and BP2 of the Prader-Willi/Angelman syndrome critical region by oligo-array CGH in a child with neurological disorder and speech impairment.Cytogenet Genome Res. 2007;116(1-2):135-40. doi: 10.1159/000097433. Cytogenet Genome Res. 2007. PMID: 17268193
-
Haploinsufficiency of Cyfip1 produces fragile X-like phenotypes in mice.PLoS One. 2012;7(8):e42422. doi: 10.1371/journal.pone.0042422. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900020 Free PMC article.
-
Clinical and genetic aspects of the 15q11.2 BP1-BP2 microdeletion disorder.J Intellect Disabil Res. 2017 Jun;61(6):568-579. doi: 10.1111/jir.12382. Epub 2017 Apr 7. J Intellect Disabil Res. 2017. PMID: 28387067 Free PMC article. Review.
-
Magnesium Supplement and the 15q11.2 BP1-BP2 Microdeletion (Burnside-Butler) Syndrome: A Potential Treatment?Int J Mol Sci. 2019 Jun 14;20(12):2914. doi: 10.3390/ijms20122914. Int J Mol Sci. 2019. PMID: 31207912 Free PMC article. Review.
Cited by
-
Cyfip1 Haploinsufficiency Increases Compulsive-Like Behavior and Modulates Palatable Food Intake in Mice: Dependence on Cyfip2 Genetic Background, Parent-of Origin, and Sex.G3 (Bethesda). 2019 Sep 4;9(9):3009-3022. doi: 10.1534/g3.119.400470. G3 (Bethesda). 2019. PMID: 31324746 Free PMC article.
-
Enhanced Prefrontal Neuronal Activity and Social Dominance Behavior in Postnatal Forebrain Excitatory Neuron-Specific Cyfip2 Knock-Out Mice.Front Mol Neurosci. 2020 Oct 29;13:574947. doi: 10.3389/fnmol.2020.574947. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33192297 Free PMC article.
-
Parent-of-Origin Effects in 15q11.2 BP1-BP2 Microdeletion (Burnside-Butler) Syndrome.Int J Mol Sci. 2019 Mar 22;20(6):1459. doi: 10.3390/ijms20061459. Int J Mol Sci. 2019. PMID: 30909440 Free PMC article.
-
Cell-autonomous reduction of CYFIP2 is insufficient to induce Alzheimer's disease-like pathologies in the hippocampal CA1 pyramidal neurons of aged mice.Anim Cells Syst (Seoul). 2023 Mar 24;27(1):93-101. doi: 10.1080/19768354.2023.2192263. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 36999135 Free PMC article.
-
Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics.Cells. 2020 Mar 31;9(4):835. doi: 10.3390/cells9040835. Cells. 2020. PMID: 32244264 Free PMC article. Review.
References
-
- Abdelmoity AT, LePichon JB, Nyp SS, Soden SE, Daniel CA, Yu S. 15q11.2 proximal imbalances associated with a diverse array of neuropsychiatric disorders and mild dysmorphic features. J Dev Behav Pediatr. 2012;33:570–6. - PubMed
-
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

