SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma
- PMID: 26475335
- PMCID: PMC4775372
- DOI: 10.1158/1078-0432.CCR-15-1522
SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma
Erratum in
-
Correction: SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma.Clin Cancer Res. 2022 Jan 15;28(2):431. doi: 10.1158/1078-0432.CCR-21-4243. Clin Cancer Res. 2022. PMID: 35045964 No abstract available.
Abstract
Purpose: Dedifferentiated liposarcoma (DDLPS) is an aggressive malignancy that can recur locally or disseminate even after multidisciplinary care. Genetically amplified and expressed MDM2, often referred to as a "hallmark" of DDLPS, mostly sustains a wild-type p53 genotype, substantiating the MDM2:p53 axis as a potential therapeutic target for DDLPS. Here, we report on the preclinical effects of SAR405838, a novel and highly selective MDM2 small-molecule inhibitor, in both in vitro and in vivo DDLPS models.
Experimental design: The therapeutic effectiveness of SAR405838 was compared with the known MDM2 antagonists Nutlin-3a and MI-219. The effects of MDM2 inhibition were assessed in both in vitro and in vivo. In vitro and in vivo microarray analyses were performed to assess differentially expressed genes induced by SAR405838, as well as the pathways that these modulated genes enriched.
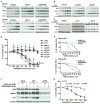
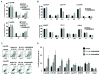
Results: SAR405838 effectively stabilized p53 and activated the p53 pathway, resulting in abrogated cellular proliferation, cell-cycle arrest, and apoptosis. Similar results were observed with Nutlin-3a and MI-219; however, significantly higher concentrations were required. In vitro effectiveness of SAR405838 activity was recapitulated in DDLPS xenograft models where significant decreases in tumorigenicity were observed. Microarray analyses revealed genes enriching the p53 signaling pathway as well as genomic stability and DNA damage following SAR405838 treatment.
Conclusions: SAR405838 is currently in early-phase clinical trials for a number of malignancies, including sarcoma, and our in vitro and in vivo results support its use as a potential therapeutic strategy for the treatment of DDLPS.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
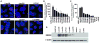


References
-
- Fletcher CDM, Bridge J, Hogendoorn P, Unni KK, Mertens F. In: World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Soft Tissue and Bone. 4. Fletcher CDM, Unni KK, Mertens F, editors. Lyon, France: IARC Press; 2013.
-
- Evans HL. Liposarcoma a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3 - PubMed
-
- Evans HL, Khurana KK, Kemp BL, Ayala AG. Heterologous Elements in the Dedifferentiated Components of Dedifferentiated Liposarcoma. Am J Clin Pathol. 1994;18:1077–182. - PubMed
-
- Ghadimi MP, Al-Zaid T, Madewell J, Peng T, Colombo C, Hoffman A, et al. Diagnosis, management, and outcome of patients with dedifferentiated liposarcoma systemic metastasis. Ann Surg Oncol. 2011;18:3762–70. - PubMed
-
- Keung EZ, Hornick JL, Bertagnolli MM, Baldini EH, Raut CP. Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery. J Am Coll Surg. 2014;218:206–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

