Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients
- PMID: 26475403
- PMCID: PMC4663163
- DOI: 10.1016/j.drugalcdep.2015.10.004
Using ethyl glucuronide in urine to detect light and heavy drinking in alcohol dependent outpatients
Abstract
Aims: This study investigated which ethyl glucuronide immunoassay (EtG-I) cutoff best detects heavy versus light drinking over five days in alcohol dependent outpatients.
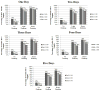
Methods: A total of 121 adults with alcohol use disorders and co-occurring psychiatric disorders took part in an alcohol treatment study. Participants provided self-reported drinking data and urine samples three times per week for 16-weeks (total samples=2761). Agreement between low (100 ng/mL, 200 ng/mL), and moderate (500 ng/mL) EtG-I cutoffs and light (women ≤3 standard drinks, men ≤4 standard drinks) and heavy drinking (women >3, men >4 standard drinks) were calculated over one to five days.
Results: The 100 ng/mL cutoff detected >76% of light drinking for two days, and 66% at five days. The 100 ng/mL cutoff detected 84% (1 day) to 79% (5 days) of heavy drinking. The 200 ng/mL cutoff detected >55% of light drinking across five days and >66% of heavy drinking across five days. A 500 ng/mL cutoff identified 68% of light drinking and 78% of heavy drinking for one day, with detection of light (2-5 days <58%) and heavy drinking (2-5 days <71%) decreasing thereafter. Relative to 100 ng/mL, the 200 ng/mL and 500 ng/mL cutoffs were less likely to result in false positives.
Conclusions: An EtG-I cutoff of 100 ng/mL is most likely to detect heavy drinking for up to five days and any drinking during the previous two days. Cutoffs of ≥500 ng/mL are likely to only detect heavy drinking during the previous day.
Keywords: Alcohol biomarkers; Assessment of cut-off; Ethyl glucuronide in urine; Heavy drinking.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Michael G. McDonell, Jordan Skalisky, Emily Leickly, Samuel Battalio, Jenny R. Nepom, and Debra Srebnik have no disclosures to report.
Sterling McPherson and John Roll have received research funding from the Bristol-Myers Squibb Foundation. This funding is in no way related to the investigation reported here. Sterling McPherson and John Roll have no disclosures to report.
Rick Ries has been on the speaker of bureaus of Janssen, Alkermes, and Reckitt Benckiser in the past three years but has no disclosures to report.
Figures
References
-
- American Psychiatric Association. DSM-IV-TR : Diagnostic And Statistical Manual Of Mental Disorders. 4. American Psychiatric Press Inc; Washington, DC: 2000. text revision.
-
- Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61:55–63. - PubMed
-
- Dahl H, Carlsson AV, Hillgren K, Helander A. Urinary ethyl glucuronide and ethyl sulfate testing for detection of recent drinking in an outpatient treatment program for alcohol and drug dependence. Alcohol Alcohol. 2011;46:278–282. - PubMed
-
- Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95(Suppl 3):S347–360. - PubMed
-
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wells EA. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107:694–708. doi: 10.1111/j.1360-0443.2011.03473.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

