Repurposing metformin: an old drug with new tricks in its binding pockets
- PMID: 26475449
- PMCID: PMC4613459
- DOI: 10.1042/BJ20150497
Repurposing metformin: an old drug with new tricks in its binding pockets
Abstract
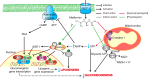
Improvements in healthcare and nutrition have generated remarkable increases in life expectancy worldwide. This is one of the greatest achievements of the modern world yet it also presents a grave challenge: as more people survive into later life, more also experience the diseases of old age, including type 2 diabetes (T2D), cardiovascular disease (CVD) and cancer. Developing new ways to improve health in the elderly is therefore a top priority for biomedical research. Although our understanding of the molecular basis of these morbidities has advanced rapidly, effective novel treatments are still lacking. Alternative drug development strategies are now being explored, such as the repurposing of existing drugs used to treat other diseases. This can save a considerable amount of time and money since the pharmacokinetics, pharmacodynamics and safety profiles of these drugs are already established, effectively enabling preclinical studies to be bypassed. Metformin is one such drug currently being investigated for novel applications. The present review provides a thorough and detailed account of our current understanding of the molecular pharmacology and signalling mechanisms underlying biguanide-protein interactions. It also focuses on the key role of the microbiota in regulating age-associated morbidities and a potential role for metformin to modulate its function. Research in this area holds the key to solving many of the mysteries of our current understanding of drug action and concerted effects to provide sustained and long-life health.
Keywords: aging; biguanides; cancer; cardiovascular disease; metformin; microbiota; phenformin; type 2 diabetes.
© 2015 Authors; published by Portland Press Limited.
Figures


References
-
- Bailey C., Day C. Metformin: its botanical background. Pract. Diabetes Int. 2004;21:115–117. doi: 10.1002/pdi.606. - DOI
-
- Watanabe C.K. Studies in the metabolic changes induced by administration of guanidine bases. J. Biol. Chem. 1918;33:253–265. I. Influence of injected guanidine hydrochloride upon blood sugar content.
-
- World Health Organization. WHO Model List of Essential Medicines. Geneva: World Health Organization; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

