Critical role of the lipid rafts in caprine herpesvirus type 1 infection in vitro
- PMID: 26475997
- PMCID: PMC7114551
- DOI: 10.1016/j.virusres.2015.10.013
Critical role of the lipid rafts in caprine herpesvirus type 1 infection in vitro
Abstract
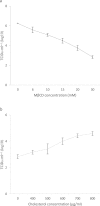
The fusion machinery for herpesvirus entry in the host cells involves the interactions of viral glycoproteins with cellular receptors, although additional viral and cellular domains are required. Extensive areas of the plasma membrane surface consist of lipid rafts organized into cholesterol-rich microdomains involved in signal transduction, protein sorting, membrane transport and in many processes of viruses infection. Because of the extraction of cholesterol leads to disorganization of lipid microdomains and to dissociation of proteins bound to the lipid rafts, we investigated the effect of cholesterol depletion by methyl-β-cyclodextrin (MβCD) on caprine herpesvirus 1 (CpHV.1) in three important phases of virus infection such as binding, entry and post-entry. MβCD treatment did not prejudice virus binding to cells, while a dose-dependent reduction of the virus yield was observed at the virus entry stage, and 30 mM MβCD reduced infectivity evidently. Treatment of MDBK after virus entry revealed a moderate inhibitory effect suggesting that cholesterol is mainly required during virus entry rather than during the post-entry stage. Alteration of the envelope lipid composition affected virus entry and a noticeable reduction in virus infectivity was detected in the presence of 15 mM MβCD. Considering that the recognition of a host cell receptor is a crucial step in the start-up phase of infection, these data are essential for the study of CpHV.1 pathogenesis. To date virus receptors for CpHV.1 have not yet been identified and further investigations are required to state that MβCD treatment affects the expression of the viral receptors.
Keywords: Cholesterol; Goat; Herpesvirus; Infectivity; Plasma membrane.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures





References
-
- Beseničar M.P., Bavdek A., Kladnik A., Maček P., Anderluh G. Kinetics of cholesterol extraction from lipid membranes by methyl-β-cyclodextrin—a surface plasmon resonance approach. Biochim. Biophys. Acta. 2008;1778:175–184. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

