BDNF in Lower Brain Parts Modifies Auditory Fiber Activity to Gain Fidelity but Increases the Risk for Generation of Central Noise After Injury
- PMID: 26476841
- PMCID: PMC5012152
- DOI: 10.1007/s12035-015-9474-x
BDNF in Lower Brain Parts Modifies Auditory Fiber Activity to Gain Fidelity but Increases the Risk for Generation of Central Noise After Injury
Abstract
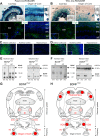
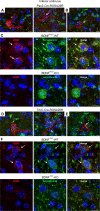
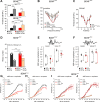
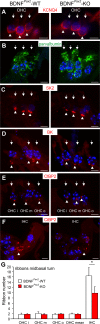
For all sensory organs, the establishment of spatial and temporal cortical resolution is assumed to be initiated by the first sensory experience and a BDNF-dependent increase in intracortical inhibition. To address the potential of cortical BDNF for sound processing, we used mice with a conditional deletion of BDNF in which Cre expression was under the control of the Pax2 or TrkC promoter. BDNF deletion profiles between these mice differ in the organ of Corti (BDNF (Pax2) -KO) versus the auditory cortex and hippocampus (BDNF (TrkC) -KO). We demonstrate that BDNF (Pax2) -KO but not BDNF (TrkC) -KO mice exhibit reduced sound-evoked suprathreshold ABR waves at the level of the auditory nerve (wave I) and inferior colliculus (IC) (wave IV), indicating that BDNF in lower brain regions but not in the auditory cortex improves sound sensitivity during hearing onset. Extracellular recording of IC neurons of BDNF (Pax2) mutant mice revealed that the reduced sensitivity of auditory fibers in these mice went hand in hand with elevated thresholds, reduced dynamic range, prolonged latency, and increased inhibitory strength in IC neurons. Reduced parvalbumin-positive contacts were found in the ascending auditory circuit, including the auditory cortex and hippocampus of BDNF (Pax2) -KO, but not of BDNF (TrkC) -KO mice. Also, BDNF (Pax2) -WT but not BDNF (Pax2) -KO mice did lose basal inhibitory strength in IC neurons after acoustic trauma. These findings suggest that BDNF in the lower parts of the auditory system drives auditory fidelity along the entire ascending pathway up to the cortex by increasing inhibitory strength in behaviorally relevant frequency regions. Fidelity and inhibitory strength can be lost following auditory nerve injury leading to diminished sensory outcome and increased central noise.
Keywords: BDNF; Central hyperactivity; High-spontaneous rate, low-threshold fibers; Homeostatic plasticity; Inferior colliculus; Sound detection threshold.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Overexpression of Isl1 under the Pax2 Promoter, Leads to Impaired Sound Processing and Increased Inhibition in the Inferior Colliculus.Int J Mol Sci. 2021 Apr 26;22(9):4507. doi: 10.3390/ijms22094507. Int J Mol Sci. 2021. PMID: 33925933 Free PMC article.
-
Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss.J Neurosci. 2012 Jun 20;32(25):8545-53. doi: 10.1523/JNEUROSCI.1247-12.2012. J Neurosci. 2012. PMID: 22723694 Free PMC article.
-
Persistent Thalamic Sound Processing Despite Profound Cochlear Denervation.Front Neural Circuits. 2016 Aug 31;10:72. doi: 10.3389/fncir.2016.00072. eCollection 2016. Front Neural Circuits. 2016. PMID: 27630546 Free PMC article.
-
The function of BDNF in the adult auditory system.Neuropharmacology. 2014 Jan;76 Pt C:719-28. doi: 10.1016/j.neuropharm.2013.05.008. Epub 2013 May 18. Neuropharmacology. 2014. PMID: 23688926 Review.
-
Is there a relationship between brain-derived neurotrophic factor for driving neuronal auditory circuits with onset of auditory function and the changes following cochlear injury or during aging?Neuroscience. 2014 Dec 26;283:26-43. doi: 10.1016/j.neuroscience.2014.07.025. Epub 2014 Jul 24. Neuroscience. 2014. PMID: 25064058 Review.
Cited by
-
Contextual auditory processing in the inferior colliculus is affected in a sex- and age-dependent manner in the valproic acid-induced rat model of autism.PLoS Biol. 2025 Aug 4;23(8):e3003309. doi: 10.1371/journal.pbio.3003309. eCollection 2025 Aug. PLoS Biol. 2025. PMID: 40758713 Free PMC article.
-
Overexpression of Isl1 under the Pax2 Promoter, Leads to Impaired Sound Processing and Increased Inhibition in the Inferior Colliculus.Int J Mol Sci. 2021 Apr 26;22(9):4507. doi: 10.3390/ijms22094507. Int J Mol Sci. 2021. PMID: 33925933 Free PMC article.
-
Age-Dependent Auditory Processing Deficits after Cochlear Synaptopathy Depend on Auditory Nerve Latency and the Ability of the Brain to Recruit LTP/BDNF.Brain Sci. 2020 Oct 6;10(10):710. doi: 10.3390/brainsci10100710. Brain Sci. 2020. PMID: 33036168 Free PMC article.
-
Detecting Noise-Induced Cochlear Synaptopathy by Auditory Brainstem Response in Tinnitus Patients With Normal Hearing Thresholds: A Meta-Analysis.Front Neurosci. 2021 Dec 20;15:778197. doi: 10.3389/fnins.2021.778197. eCollection 2021. Front Neurosci. 2021. PMID: 34987358 Free PMC article.
-
Guanylyl Cyclase A/cGMP Signaling Slows Hidden, Age- and Acoustic Trauma-Induced Hearing Loss.Front Aging Neurosci. 2020 Apr 9;12:83. doi: 10.3389/fnagi.2020.00083. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32327991 Free PMC article.
References
-
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

