TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer
- PMID: 26477313
- PMCID: PMC6310059
- DOI: 10.1038/onc.2015.253
TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer
Abstract
The BRAF(V600E) mutation is found in approximately 40% of papillary thyroid cancers (PTC). Mice with thyroid-specific expression of Braf(V600E) (TPO-Braf(V600E)) develop PTC rapidly with high levels of serum thyroid-stimulating hormone (TSH). It is unclear to what extent the elevated TSH contributes to tumor progression. To investigate the progression of Braf(V600E)-induced PTC (BVE-PTC) under normal TSH, we transplanted BVE-PTC tumors subcutaneously into nude and TPO-Braf(WT) mice. Regression of the transplanted tumors was observed in both nude and TPO-Braf(WT) mice. They were surrounded by heavy lymphocyte infiltration and oncogene-induced senescence (OIS) was demonstrated by strong β-gal staining and absence of Ki-67 expression. In contrast, BVE-PTC transplants continued to grow when transplanted into TPO-Braf(V600E) mice. The expression of Trp53 was increased in tumor transplants undergoing OIS. Trp53 inactivation reversed OIS and enabled tumor transplants to grow in nude mice with characteristic cell morphology of anaplastic thyroid cancer (ATC). PTC-to-ATC transformation was also observed in primary BVE-PTC tumors. ATC cells derived from Trp53 knockout tumors had increased PI3K/AKT signaling and became resistant to Braf(V600E) inhibitor PLX4720, which could be overcome by combined treatment of PI3K inhibitor LY294002 and PLX4720. In conclusion, BVE-PTC progression could be contained via p53-dependent OIS and TSH is a major disruptor of this balance. Simultaneous targeting of both MAPK and PI3K/AKT pathways offer a better therapeutic outcome against ATC. The current study reinforces the importance of rigorous control of serum TSH in PTC patients.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
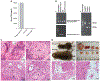
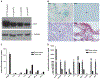
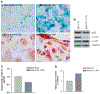


Similar articles
-
Cyp24a1 Attenuation Limits Progression of BrafV600E -Induced Papillary Thyroid Cancer Cells and Sensitizes Them to BRAFV600E Inhibitor PLX4720.Cancer Res. 2017 Apr 15;77(8):2161-2172. doi: 10.1158/0008-5472.CAN-16-2066. Epub 2017 Feb 27. Cancer Res. 2017. PMID: 28242615 Free PMC article.
-
p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer.Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):E1600-9. doi: 10.1073/pnas.1404357111. Epub 2014 Apr 7. Proc Natl Acad Sci U S A. 2014. PMID: 24711431 Free PMC article.
-
β-Catenin Attenuation Inhibits Tumor Growth and Promotes Differentiation in a BRAFV600E-Driven Thyroid Cancer Animal Model.Mol Cancer Ther. 2021 Sep;20(9):1603-1613. doi: 10.1158/1535-7163.MCT-21-0037. Epub 2021 Jun 17. Mol Cancer Ther. 2021. PMID: 34224366
-
Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations.Cancer. 2005 Jun 1;103(11):2261-8. doi: 10.1002/cncr.21073. Cancer. 2005. PMID: 15880523 Review.
-
BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications.J Chin Med Assoc. 2010 Mar;73(3):113-28. doi: 10.1016/S1726-4901(10)70025-3. J Chin Med Assoc. 2010. PMID: 20230995 Review.
Cited by
-
Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention.Diabetes Metab Syndr Obes. 2024 Feb 16;17:809-823. doi: 10.2147/DMSO.S450321. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38380275 Free PMC article. Review.
-
Comprehensive analysis of cellular senescence and immune microenvironment in papillary thyroid carcinoma.Aging (Albany NY). 2024 Feb 7;16(3):2866-2886. doi: 10.18632/aging.205520. Epub 2024 Feb 7. Aging (Albany NY). 2024. PMID: 38329430 Free PMC article.
-
Papillary thyroid carcinoma with Hashimoto's thyroiditis: impact and correlation.Front Endocrinol (Lausanne). 2025 Apr 11;16:1512417. doi: 10.3389/fendo.2025.1512417. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40290312 Free PMC article. Review.
-
Tissue specificity of oncogenic BRAF targeted to lung and thyroid through a shared lineage factor.iScience. 2023 Jun 8;26(7):107071. doi: 10.1016/j.isci.2023.107071. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534159 Free PMC article.
-
The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy.Cells. 2021 May 1;10(5):1082. doi: 10.3390/cells10051082. Cells. 2021. PMID: 34062862 Free PMC article. Review.
References
-
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006; 295: 2164–2167. - PubMed
-
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer 1998; 83: 2638–2648. - PubMed
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19: 1167–1214. - PubMed
-
- Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001; 86: 1447–1463. - PubMed
-
- Hollenbeak CS, Boltz MM, Schaefer EW, Saunders BD, Goldenberg D. Recurrence of differentiated thyroid cancer in the elderly. Eur J Endocrinol 2013; 168: 549–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

