Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability
- PMID: 26477653
- PMCID: PMC4692516
- DOI: 10.1016/j.neuroimage.2015.10.018
Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability
Abstract
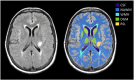
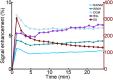
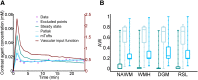
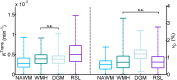
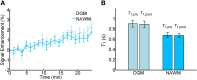
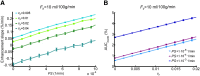
There is evidence that subtle breakdown of the blood-brain barrier (BBB) is a pathophysiological component of several diseases, including cerebral small vessel disease and some dementias. Dynamic contrast-enhanced MRI (DCE-MRI) combined with tracer kinetic modelling is widely used for assessing permeability and perfusion in brain tumours and body tissues where contrast agents readily accumulate in the extracellular space. However, in diseases where leakage is subtle, the optimal approach for measuring BBB integrity is likely to differ since the magnitude and rate of enhancement caused by leakage are extremely low; several methods have been reported in the literature, yielding a wide range of parameters even in healthy subjects. We hypothesised that the Patlak model is a suitable approach for measuring low-level BBB permeability with low temporal resolution and high spatial resolution and brain coverage, and that normal levels of scanner instability would influence permeability measurements. DCE-MRI was performed in a cohort of mild stroke patients (n=201) with a range of cerebral small vessel disease severity. We fitted these data to a set of nested tracer kinetic models, ranking their performance according to the Akaike information criterion. To assess the influence of scanner drift, we scanned 15 healthy volunteers that underwent a "sham" DCE-MRI procedure without administration of contrast agent. Numerical simulations were performed to investigate model validity and the effect of scanner drift. The Patlak model was found to be most appropriate for fitting low-permeability data, and the simulations showed vp and K(Trans) estimates to be reasonably robust to the model assumptions. However, signal drift (measured at approximately 0.1% per minute and comparable to literature reports in other settings) led to systematic errors in calculated tracer kinetic parameters, particularly at low permeabilities. Our findings justify the growing use of the Patlak model in low-permeability states, which has the potential to provide valuable information regarding BBB integrity in a range of diseases. However, absolute values of the resulting tracer kinetic parameters should be interpreted with extreme caution, and the size and influence of signal drift should be measured where possible.
Keywords: Blood–brain barrier; Cerebral small vessel disease; Dynamic contrast-enhanced MRI; Tracer kinetic modelling.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ahearn T.S., Staff R.T., Redpath T.W., Semple S.I.K. The use of the Levenberg–Marquardt curve-fitting algorithm in pharmacokinetic modelling of DCE-MRI data. Phys. Med. Biol. 2005;50:N85–N92. - PubMed
-
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723.
-
- Armitage P., Behrenbruch C., Brady M., Moore N. Extracting and visualizing physiological parameters using dynamic contrast-enhanced magnetic resonance imaging of the breast. Med. Image Anal. 2005;9:315–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

