Laminar microvascular transit time distribution in the mouse somatosensory cortex revealed by Dynamic Contrast Optical Coherence Tomography
- PMID: 26477654
- PMCID: PMC4691378
- DOI: 10.1016/j.neuroimage.2015.10.017
Laminar microvascular transit time distribution in the mouse somatosensory cortex revealed by Dynamic Contrast Optical Coherence Tomography
Abstract
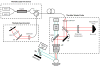
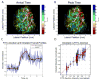
The transit time distribution of blood through the cerebral microvasculature both constrains oxygen delivery and governs the kinetics of neuroimaging signals such as blood-oxygen-level-dependent functional Magnetic Resonance Imaging (BOLD fMRI). However, in spite of its importance, capillary transit time distribution has been challenging to quantify comprehensively and efficiently at the microscopic level. Here, we introduce a method, called Dynamic Contrast Optical Coherence Tomography (DyC-OCT), based on dynamic cross-sectional OCT imaging of an intravascular tracer as it passes through the field-of-view. Quantitative transit time metrics are derived from temporal analysis of the dynamic scattering signal, closely related to tracer concentration. Since DyC-OCT does not require calibration of the optical focus, quantitative accuracy is achieved even deep in highly scattering brain tissue where the focal spot degrades. After direct validation of DyC-OCT against dilution curves measured using a fluorescent plasma label in surface pial vessels, we used DyC-OCT to investigate the transit time distribution in microvasculature across the entire depth of the mouse somatosensory cortex. Laminar trends were identified, with earlier transit times and less heterogeneity in the middle cortical layers. The early transit times in the middle cortical layers may explain, at least in part, the early BOLD fMRI onset times observed in these layers. The layer-dependencies in heterogeneity may help explain how a single vascular supply manages to deliver oxygen to individual cortical layers with diverse metabolic needs.
Keywords: Blood flow; Dynamic contrast; Functional magnetic resonance imaging; Hemodynamics; Optical coherence tomography; Transit time.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Dynamic Contrast Optical Coherence Tomography reveals laminar microvascular hemodynamics in the mouse neocortex in vivo.Neuroimage. 2019 Nov 15;202:116067. doi: 10.1016/j.neuroimage.2019.116067. Epub 2019 Aug 5. Neuroimage. 2019. PMID: 31394180 Free PMC article.
-
Dynamic contrast optical coherence tomography images transit time and quantifies microvascular plasma volume and flow in the retina and choriocapillaris.Biomed Opt Express. 2016 Sep 27;7(10):4289-4312. doi: 10.1364/BOE.7.004289. eCollection 2016 Oct 1. Biomed Opt Express. 2016. PMID: 27867732 Free PMC article.
-
Optical Coherence Tomography angiography reveals laminar microvascular hemodynamics in the rat somatosensory cortex during activation.Neuroimage. 2014 Nov 15;102 Pt 2(0 2):393-406. doi: 10.1016/j.neuroimage.2014.08.004. Epub 2014 Aug 8. Neuroimage. 2014. PMID: 25111471 Free PMC article.
-
Dynamic contrast optical coherence tomography (DyC-OCT) for label-free live cell imaging.Commun Biol. 2024 Mar 6;7(1):278. doi: 10.1038/s42003-024-05973-5. Commun Biol. 2024. PMID: 38448627 Free PMC article. Review.
-
Submillimeter-resolution fMRI: Toward understanding local neural processing.Prog Brain Res. 2016;225:123-52. doi: 10.1016/bs.pbr.2016.03.003. Epub 2016 Apr 1. Prog Brain Res. 2016. PMID: 27130414 Review.
Cited by
-
Investigation of artifacts in retinal and choroidal OCT angiography with a contrast agent.Biomed Opt Express. 2018 Feb 6;9(3):1020-1040. doi: 10.1364/BOE.9.001020. eCollection 2018 Mar 1. Biomed Opt Express. 2018. PMID: 29541501 Free PMC article.
-
Visible light optical coherence microscopy of the brain with isotropic femtoliter resolution in vivo.Opt Lett. 2018 Jan 15;43(2):198-201. doi: 10.1364/OL.43.000198. Opt Lett. 2018. PMID: 29328237 Free PMC article.
-
Seizure epicenter depth and translaminar field potential synchrony underlie complex variations in tissue oxygenation during ictal initiation.Neuroimage. 2018 May 1;171:165-175. doi: 10.1016/j.neuroimage.2017.12.088. Epub 2017 Dec 30. Neuroimage. 2018. PMID: 29294386 Free PMC article.
-
Optical microangiography reveals temporal and depth-resolved hemodynamic change in mouse barrel cortex during whisker stimulation.J Biomed Opt. 2020 Sep;25(9):096005. doi: 10.1117/1.JBO.25.9.096005. J Biomed Opt. 2020. PMID: 32945154 Free PMC article.
-
Visualization of neuritic plaques in Alzheimer's disease by polarization-sensitive optical coherence microscopy.Sci Rep. 2017 Mar 6;7:43477. doi: 10.1038/srep43477. Sci Rep. 2017. PMID: 28262719 Free PMC article.
References
-
- Kety SS. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3(1):1–41. - PubMed
-
- Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension a possible mechanism for the participation of oxygen in local regulation of blood flow. Circulation Research. 1970;27(5):669–678. - PubMed
-
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge University Press; 2002.
-
- Marota JJ, et al. Investigation of the early response to rat forepaw stimulation. Magn Reson Med. 1999;41(2):247–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

