Seasonal Expression of Prolactin Receptor in the Scented Gland of Male Muskrat (Ondatra zibethicus)
- PMID: 26477851
- PMCID: PMC4609948
- DOI: 10.1038/srep15036
Seasonal Expression of Prolactin Receptor in the Scented Gland of Male Muskrat (Ondatra zibethicus)
Abstract
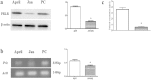
Prolactin (PRL) has numerous actions in mammalian biological systems including mammary development and biological processes. The aim of this study was to investigate the seasonal changes of prolactin receptor (PRLR) expression in the scented gland of muskrat during the breeding and nonbreeding seasons. Histologically, glandular cells, interstitial cells and excretory tubules were identified in the scented glands in both seasons, whereas epithelial cells were sparse in the nonbreeding season. PRLR was observed in glandular cells of scented glands during the breeding and nonbreeding seasons with stronger immunostaining during the breeding season. Consistent with the immunohistochemical results, both the mean of protein and mRNA levels of PRLR were higher in the scented glands of the breeding season, and relatively lower level in the nonbreeding season. In addition, differential seasonal changes were also detected in the expression profile of microRNAs (miRNAs) in the scented gland of muskrat. Besides, plasma PRL concentration was remarkably higher in the breeding season than that in the nonbreeding season. These results suggested that muskrat scented gland was the direct target organ of PRL, and stronger expression of PRLR in scented glands during the breeding season indicated that PRL may directly regulate scented glandular function of the muskrats.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Seasonal expressions of prolactin, prolactin receptor and STAT5 in the scented glands of the male muskrats (Ondatra zibethicus).Eur J Histochem. 2019 Jan 17;63(1):2991. doi: 10.4081/ejh.2019.2991. Eur J Histochem. 2019. PMID: 30652434 Free PMC article.
-
Seasonal expression of androgen receptor in scented gland of muskrat (Ondatra zibethicus).Gen Comp Endocrinol. 2014 Aug 1;204:1-7. doi: 10.1016/j.ygcen.2014.04.031. Epub 2014 May 10. Gen Comp Endocrinol. 2014. PMID: 24818970
-
Seasonal expression of P450c17 and 5α-reductase-2 in the scented gland of male muskrats (Ondatra zibethicus).Gen Comp Endocrinol. 2017 Dec 1;254:60-67. doi: 10.1016/j.ygcen.2017.09.015. Epub 2017 Sep 14. Gen Comp Endocrinol. 2017. PMID: 28919450
-
Prolactin (PRL) and prolactin receptor (PRLR) genes and their role in poultry production traits.Folia Biol (Krakow). 2014;62(1):1-8. doi: 10.3409/fb62_1.1. Folia Biol (Krakow). 2014. PMID: 24745142 Review.
-
Triennial Lactation Symposium: Prolactin: The multifaceted potentiator of mammary growth and function.J Anim Sci. 2012 May;90(5):1674-86. doi: 10.2527/jas.2011-4682. Epub 2011 Dec 28. J Anim Sci. 2012. PMID: 22205663 Review.
Cited by
-
Seasonal Expression of Oxytocin and Oxytocin Receptor in the Scented Gland of Male Muskrat (Ondatra zibethicus).Sci Rep. 2017 Nov 30;7(1):16627. doi: 10.1038/s41598-017-16973-3. Sci Rep. 2017. PMID: 29192229 Free PMC article.
-
Seasonal Changes in the Structure and Function of Gut Microbiota in the Muskrat (Ondatra zibethicus).Metabolites. 2023 Feb 9;13(2):248. doi: 10.3390/metabo13020248. Metabolites. 2023. PMID: 36837868 Free PMC article.
-
Seasonal expressions of prolactin, prolactin receptor and STAT5 in the scented glands of the male muskrats (Ondatra zibethicus).Eur J Histochem. 2019 Jan 17;63(1):2991. doi: 10.4081/ejh.2019.2991. Eur J Histochem. 2019. PMID: 30652434 Free PMC article.
-
Exploring the impact of chia seeds and matcha green tea on gene expression related to the puberty pathway in growing male New Zealand white rabbits.Trop Anim Health Prod. 2025 Apr 2;57(3):152. doi: 10.1007/s11250-025-04391-x. Trop Anim Health Prod. 2025. PMID: 40172761 Free PMC article.
-
Chromosome-level Genome of the Muskrat (Ondatra zibethicus).Genome Biol Evol. 2022 Oct 7;14(10):evac138. doi: 10.1093/gbe/evac138. Genome Biol Evol. 2022. PMID: 36108314 Free PMC article.
References
-
- Freeman M. E., Kanyicska B., Lerant A. & Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80, 1523–1631 (2000). - PubMed
-
- Clapp C., Thebault S. & Martinez de la Escalera G. Role of prolactin and vasoinhibins in the regulation of vascular function in mammary gland. J Mammary Gland Biol Neoplasia 13, 55–67 (2008). - PubMed
-
- Bole-Feysot C., Goffin V., Edery M., Binart N. & Kelly P. A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19, 225–268 (1998). - PubMed
-
- Bernichtein S., Touraine P. & Goffin V. New concepts in prolactin biology. J Endocrinol 206, 1–11 (2010). - PubMed
-
- Bachelot A. & Binart N. Reproductive role of prolactin. Reproduction 133, 361–369 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

