Evolving new protein-protein interaction specificity through promiscuous intermediates
- PMID: 26478181
- PMCID: PMC4623991
- DOI: 10.1016/j.cell.2015.09.055
Evolving new protein-protein interaction specificity through promiscuous intermediates
Abstract
Interacting proteins typically coevolve, and the identification of coevolving amino acids can pinpoint residues required for interaction specificity. This approach often assumes that an interface-disrupting mutation in one protein drives selection of a compensatory mutation in its partner during evolution. However, this model requires a non-functional intermediate state prior to the compensatory change. Alternatively, a mutation in one protein could first broaden its specificity, allowing changes in its partner, followed by a specificity-restricting mutation. Using bacterial toxin-antitoxin systems, we demonstrate the plausibility of this second, promiscuity-based model. By screening large libraries of interface mutants, we show that toxins and antitoxins with high specificity are frequently connected in sequence space to more promiscuous variants that can serve as intermediates during a reprogramming of interaction specificity. We propose that the abundance of promiscuous variants promotes the expansion and diversification of toxin-antitoxin systems and other paralogous protein families during evolution.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
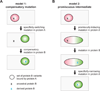




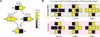
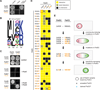
Comment in
-
Evolutionary reprograming of protein-protein interaction specificity.Cell. 2015 Oct 22;163(3):535-7. doi: 10.1016/j.cell.2015.10.010. Epub 2015 Oct 22. Cell. 2015. PMID: 26496596
-
Evolution: Mix and re-match.Nat Rev Genet. 2015 Dec;16(12):686-7. doi: 10.1038/nrg4035. Epub 2015 Oct 27. Nat Rev Genet. 2015. PMID: 26503798 No abstract available.
References
-
- Aharoni A, Gaidukov L, Khersonsky O, Mc QGS, Roodveldt C, Tawfik DS. The 'evolvability' of promiscuous protein functions. Nat Genet. 2005;37:73–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

