Comparison of acute non-visual bright light responses in patients with optic nerve disease, glaucoma and healthy controls
- PMID: 26478261
- PMCID: PMC4609937
- DOI: 10.1038/srep15185
Comparison of acute non-visual bright light responses in patients with optic nerve disease, glaucoma and healthy controls
Abstract
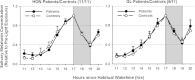
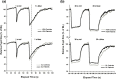
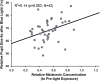
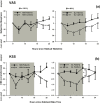
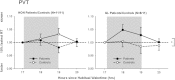
This study examined the effect of optic nerve disease, hence retinal ganglion cell loss, on non-visual functions related to melanopsin signalling. Test subjects were patients with bilateral visual loss and optic atrophy from either hereditary optic neuropathy (n = 11) or glaucoma (n = 11). We measured melatonin suppression, subjective sleepiness and cognitive functions in response to bright light exposure in the evening. We also quantified the post-illumination pupil response to a blue light stimulus. All results were compared to age-matched controls (n = 22). Both groups of patients showed similar melatonin suppression when compared to their controls. Greater melatonin suppression was intra-individually correlated to larger post-illumination pupil response in patients and controls. Only the glaucoma patients demonstrated a relative attenuation of their pupil response. In addition, they were sleepier with slower reaction times during nocturnal light exposure. In conclusion, glaucomatous, but not hereditary, optic neuropathy is associated with reduced acute light effects. At mild to moderate stages of disease, this is detected only in the pupil function and not in responses conveyed via the retinohypothalamic tract such as melatonin suppression.
Conflict of interest statement
Dr Kawasaki has received book royalties from Cambridge University Press.
Figures

References
-
- Berson D. M., Dunn F. A. & Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002). - PubMed
-
- Ruby N. et al. Role of melanopsin in circadian responses to light. Science 298, 2211–2213 (2002). - PubMed
-
- Lucas R. J. et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

