A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination
- PMID: 26478840
- PMCID: PMC4603406
- DOI: 10.1002/open.201500056
A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination
Abstract
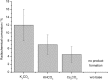
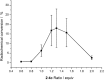
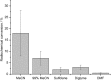
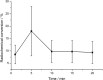
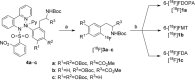
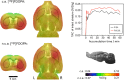
Recently a novel method for the preparation of (18)F-labeled arenes via oxidative [(18)F]fluorination of easily accessible and sufficiently stable nickel complexes with [(18)F]fluoride under exceptionally mild reaction conditions was published. The suitability of this procedure for the routine preparation of clinically relevant positron emission tomography (PET) tracers, 6-[(18)F]fluorodopamine (6-[(18)F]FDA), 6-[(18)F]fluoro-l-DOPA (6-[(18)F]FDOPA) and 6-[(18)F]fluoro-m-tyrosine (6-[(18)F]FMT), was evaluated. The originally published base-free method was inoperative. However, a "low base" protocol afforded protected radiolabeled intermediates in radiochemical conversions (RCCs) of 5-18 %. The subsequent deprotection step proceeded almost quantitatively (>95 %). The simple one-pot two-step procedure allowed the preparation of clinical doses of 6-[(18)F]FDA and 6-[(18)F]FDOPA within 50 min (12 and 7 % radiochemical yield, respectively). In an unilateral rat model of Parkinsons disease, 6-[(18)F]FDOPA with high specific activity (175 GBq μmol(-1)) prepared using the described nickel-mediated radiofluorination was compared to 6-[(18)F]FDOPA with low specific activity (30 MBq μmol(-1)) produced via conventional electrophilic radiofluorination. Unexpectedly both tracer variants displayed very similar in vivo properties with respect to signal-to-noise ratio and brain distribution, and consequently, the quality of the obtained PET images was almost identical.
Keywords: [18F]fluoride; nucleophilic aromatic substitution; positron emission tomography (PET); radiopharmaceuticals; radiosynthesis.
Figures
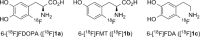
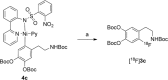
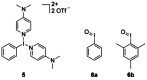
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

