Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons(1,2,3)
- PMID: 26478909
- PMCID: PMC4603253
- DOI: 10.1523/ENEURO.0069-15.2015
Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons(1,2,3)
Abstract
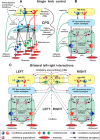
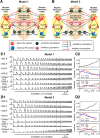
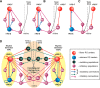
The organization of neural circuits that form the locomotor central pattern generator (CPG) and provide flexor-extensor and left-right coordination of neuronal activity remains largely unknown. However, significant progress has been made in the molecular/genetic identification of several types of spinal interneurons, including V0 (V0D and V0V subtypes), V1, V2a, V2b, V3, and Shox2, among others. The possible functional roles of these interneurons can be suggested from changes in the locomotor pattern generated in mutant mice lacking particular neuron types. Computational modeling of spinal circuits may complement these studies by bringing together data from different experimental studies and proposing the possible connectivity of these interneurons that may define rhythm generation, flexor-extensor interactions on each side of the cord, and commissural interactions between left and right circuits. This review focuses on the analysis of potential architectures of spinal circuits that can reproduce recent results and suggest common explanations for a series of experimental data on genetically identified spinal interneurons, including the consequences of their genetic ablation, and provides important insights into the organization of the spinal CPG and neural control of locomotion.
Keywords: central pattern generator; computational modeling; flexor–extensor coordination; genetically identified neurons; left–right coordination; locomotion; spinal cord.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
