Developing Extracellular Matrix Technology to Treat Retinal or Optic Nerve Injury(1,2,3)
- PMID: 26478910
- PMCID: PMC4603254
- DOI: 10.1523/ENEURO.0077-15.2015
Developing Extracellular Matrix Technology to Treat Retinal or Optic Nerve Injury(1,2,3)
Abstract
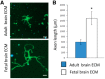
Adult mammalian CNS neurons often degenerate after injury, leading to lost neurologic functions. In the visual system, retinal or optic nerve injury often leads to retinal ganglion cell axon degeneration and irreversible vision loss. CNS axon degeneration is increasingly linked to the innate immune response to injury, which leads to tissue-destructive inflammation and scarring. Extracellular matrix (ECM) technology can reduce inflammation, while increasing functional tissue remodeling, over scarring, in various tissues and organs, including the peripheral nervous system. However, applying ECM technology to CNS injuries has been limited and virtually unstudied in the visual system. Here we discuss advances in deriving fetal CNS-specific ECMs, like fetal porcine brain, retina, and optic nerve, and fetal non-CNS-specific ECMs, like fetal urinary bladder, and the potential for using tissue-specific ECMs to treat retinal or optic nerve injuries in two platforms. The first platform is an ECM hydrogel that can be administered as a retrobulbar, periocular, or even intraocular injection. The second platform is an ECM hydrogel and polymer "biohybrid" sheet that can be readily shaped and wrapped around a nerve. Both platforms can be tuned mechanically and biochemically to deliver factors like neurotrophins, immunotherapeutics, or stem cells. Since clinical CNS therapies often use general anti-inflammatory agents, which can reduce tissue-destructive inflammation but also suppress tissue-reparative immune system functions, tissue-specific, ECM-based devices may fill an important need by providing naturally derived, biocompatible, and highly translatable platforms that can modulate the innate immune response to promote a positive functional outcome.
Keywords: ECM; axon regeneration; immunotherapy; regenerative medicine; retinal ganglion cell.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Regeneration of axons in the visual system.Restor Neurol Neurosci. 2008;26(2-3):147-74. Restor Neurol Neurosci. 2008. PMID: 18820408 Review.
-
Quantitative iTRAQ analysis of retinal ganglion cell degeneration after optic nerve crush.J Proteome Res. 2011 Aug 5;10(8):3344-62. doi: 10.1021/pr2004055. Epub 2011 Jun 29. J Proteome Res. 2011. PMID: 21627321
-
Regeneration of axons from adult rat retinal ganglion cells on cultured Schwann cells is not dependent on basal lamina.Glia. 1991;4(1):46-55. doi: 10.1002/glia.440040106. Glia. 1991. PMID: 1828786
-
Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth.Brain. 2009 Nov;132(Pt 11):3102-21. doi: 10.1093/brain/awp240. Epub 2009 Sep 25. Brain. 2009. PMID: 19783665
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
Cited by
-
Tenascins in Retinal and Optic Nerve Neurodegeneration.Front Integr Neurosci. 2017 Oct 23;11:30. doi: 10.3389/fnint.2017.00030. eCollection 2017. Front Integr Neurosci. 2017. PMID: 29109681 Free PMC article. Review.
-
Fabrication of transparent hemispherical 3D nanofibrous scaffolds with radially aligned patterns via a novel electrospinning method.Sci Rep. 2018 Feb 21;8(1):3424. doi: 10.1038/s41598-018-21618-0. Sci Rep. 2018. PMID: 29467436 Free PMC article.
-
Extracellular-Matrix Mechanics Regulate the Ocular Physiological and Pathological Activities.J Ophthalmol. 2023 Jul 22;2023:7626920. doi: 10.1155/2023/7626920. eCollection 2023. J Ophthalmol. 2023. PMID: 37521908 Free PMC article. Review.
-
Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma.Front Pharmacol. 2021 Nov 2;12:764709. doi: 10.3389/fphar.2021.764709. eCollection 2021. Front Pharmacol. 2021. PMID: 34795592 Free PMC article.
-
Collagen mimetic peptide repair of the corneal nerve bed in a mouse model of dry eye disease.Front Neurosci. 2023 May 16;17:1148950. doi: 10.3389/fnins.2023.1148950. eCollection 2023. Front Neurosci. 2023. PMID: 37260844 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
