6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling
- PMID: 26479318
- PMCID: PMC4628560
- DOI: 10.1038/ncb3255
6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling
Abstract
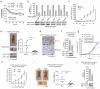
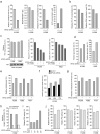
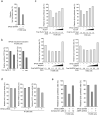
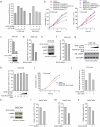
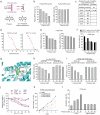
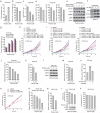
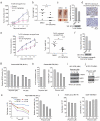
The oxidative pentose phosphate pathway (PPP) contributes to tumour growth, but the precise contribution of 6-phosphogluconate dehydrogenase (6PGD), the third enzyme in this pathway, to tumorigenesis remains unclear. We found that suppression of 6PGD decreased lipogenesis and RNA biosynthesis and elevated ROS levels in cancer cells, attenuating cell proliferation and tumour growth. 6PGD-mediated production of ribulose-5-phosphate (Ru-5-P) inhibits AMPK activation by disrupting the active LKB1 complex, thereby activating acetyl-CoA carboxylase 1 and lipogenesis. Ru-5-P and NADPH are thought to be precursors in RNA biosynthesis and lipogenesis, respectively; thus, our findings provide an additional link between the oxidative PPP and lipogenesis through Ru-5-P-dependent inhibition of LKB1-AMPK signalling. Moreover, we identified and developed 6PGD inhibitors, physcion and its derivative S3, that effectively inhibited 6PGD, cancer cell proliferation and tumour growth in nude mice xenografts without obvious toxicity, suggesting that 6PGD could be an anticancer target.
Figures

Similar articles
-
γ-6-Phosphogluconolactone, a Byproduct of the Oxidative Pentose Phosphate Pathway, Contributes to AMPK Activation through Inhibition of PP2A.Mol Cell. 2019 Dec 19;76(6):857-871.e9. doi: 10.1016/j.molcel.2019.09.007. Epub 2019 Oct 2. Mol Cell. 2019. PMID: 31586547 Free PMC article.
-
Inhibiting 6-phosphogluconate dehydrogenase enhances chemotherapy efficacy in cervical cancer via AMPK-independent inhibition of RhoA and Rac1.Clin Transl Oncol. 2019 Apr;21(4):404-411. doi: 10.1007/s12094-018-1937-x. Epub 2018 Sep 4. Clin Transl Oncol. 2019. PMID: 30182212
-
Inhibition of 6-phosphogluconate dehydrogenase suppresses esophageal squamous cell carcinoma growth and enhances the anti-tumor effects of metformin via the AMPK/mTOR pathway.Mol Cancer. 2025 Mar 26;24(1):97. doi: 10.1186/s12943-025-02302-0. Mol Cancer. 2025. PMID: 40140842 Free PMC article.
-
6-Phosphogluconate dehydrogenase fuels multiple aspects of cancer cells: From cancer initiation to metastasis and chemoresistance.Biofactors. 2020 Jul;46(4):550-562. doi: 10.1002/biof.1624. Epub 2020 Feb 10. Biofactors. 2020. PMID: 32039535 Review.
-
The LKB1/AMPK polarity pathway.FEBS Lett. 2011 Apr 6;585(7):981-5. doi: 10.1016/j.febslet.2010.12.025. Epub 2010 Dec 23. FEBS Lett. 2011. PMID: 21185289 Review.
Cited by
-
Inducing disulfidptosis in tumors:potential pathways and significance.MedComm (2020). 2024 Oct 15;5(11):e791. doi: 10.1002/mco2.791. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39415848 Free PMC article. Review.
-
PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers.Oncogene. 2019 Oct;38(40):6662-6677. doi: 10.1038/s41388-019-0908-1. Epub 2019 Aug 7. Oncogene. 2019. PMID: 31391551 Free PMC article.
-
The Tumor Suppressor Kinase LKB1: Metabolic Nexus.Front Cell Dev Biol. 2022 Apr 28;10:881297. doi: 10.3389/fcell.2022.881297. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573694 Free PMC article. Review.
-
DGKA Provides Platinum Resistance in Ovarian Cancer Through Activation of c-JUN-WEE1 Signaling.Clin Cancer Res. 2020 Jul 15;26(14):3843-3855. doi: 10.1158/1078-0432.CCR-19-3790. Epub 2020 Apr 27. Clin Cancer Res. 2020. PMID: 32341033 Free PMC article.
-
Effects of Timolol Treatment on Pancreatic Antioxidant Enzymes in Streptozotocin-induced Diabetic Rats: An Experimental and Computational Study.J Med Biochem. 2019 May 11;38(3):306-316. doi: 10.2478/jomb-2018-0034. eCollection 2019 Jul. J Med Biochem. 2019. PMID: 31156341 Free PMC article.
References
-
- Warburg O. On the origin of cancer cells. Science. Science. Science. Science. Science. 1956;123:309–314. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews. Cancer. 2011;11:85–95. - PubMed
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. - PubMed
-
- Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

