Considering Eligibility for Studies of Deep Brain Stimulation for Treatment-Resistant Depression: Insights From a Clinical Trial in Unipolar and Bipolar Depression
- PMID: 26479487
- PMCID: PMC4834065
- DOI: 10.1097/YCT.0000000000000281
Considering Eligibility for Studies of Deep Brain Stimulation for Treatment-Resistant Depression: Insights From a Clinical Trial in Unipolar and Bipolar Depression
Abstract
Background: While electroconvulsive therapy (ECT) is the most effective treatment for major depression (major depressive disorder [MDD]), deep brain stimulation (DBS) has shown efficacy in patients who have not received benefit from ECT. Studies of DBS are small, and a better understanding of which eligibility criteria lead to exclusion may help achieve a more appropriate balance between scientific rigor and generalizability in future trials. We assessed the rate and reasons for exclusion from a study of DBS for treatment-resistant MDD and bipolar type II (BPII) depression.
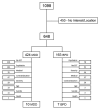
Methods: One thousand ninety-eight adults were screened for a study of DBS for MDD or BPII. Reasons for exclusion were documented. Descriptive statistics were calculated for each reason for exclusion for the entire sample as well as the self-reported MDD and BPII subgroups.
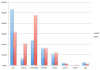
Results: Ninety-eight percent (98%) of patients screened were excluded. Exclusion due to lack of interest or inability to relocate to the study site was high (41%). Following this, primary reasons for exclusion were lack of prior ECT and presence of psychiatric/general medical comorbidity. Patients with MDD were more likely to be excluded because of inadequate ECT, whereas patients with BPII depression were more likely to be excluded for comorbid psychiatric diagnoses and not meeting minimum severity criteria.
Conclusions: A surprisingly high number of potential participants were excluded because of lack of adequate ECT. This suggests that many patients self-identifying as "treatment resistant" have not truly exhausted available, evidence-based treatments. Overall exclusion rate was high, with key differences in exclusion reasons between the MDD and BPII subgroups. These findings can inform design of future clinical trials for treatment-resistant unipolar and bipolar depression.Clinicaltrials.gov Identifier: NCT00367003.
Figures
References
-
- The UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. The Lancet. 2003;361:799–808. - PubMed
-
- Avery D. Transcranial magnetic stimulation in the treatment of depression. Essent Psychopharmacol. 2001;4(1):37–48. - PubMed
-
- Avery DH, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. J Nerv Ment Dis. 1999;187(2):114–7. - PubMed
-
- Avery DH, et al. 59th Annual Meeting of the Society of Biological Psychiatry. New York, NY.: 2004. Repetitive transcranial magnetic stimulation (rTMS) is clinically effective in treatment-resistant major depression.
-
- Avery DH, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008;69(3):441–51. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

