Synaptic Efficacy as a Function of Ionotropic Receptor Distribution: A Computational Study
- PMID: 26480028
- PMCID: PMC4610697
- DOI: 10.1371/journal.pone.0140333
Synaptic Efficacy as a Function of Ionotropic Receptor Distribution: A Computational Study
Abstract
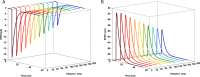
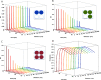
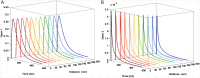
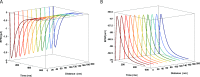
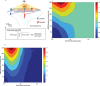
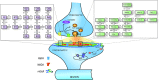
Glutamatergic synapses are the most prevalent functional elements of information processing in the brain. Changes in pre-synaptic activity and in the function of various post-synaptic elements contribute to generate a large variety of synaptic responses. Previous studies have explored postsynaptic factors responsible for regulating synaptic strength variations, but have given far less importance to synaptic geometry, and more specifically to the subcellular distribution of ionotropic receptors. We analyzed the functional effects resulting from changing the subsynaptic localization of ionotropic receptors by using a hippocampal synaptic computational framework. The present study was performed using the EONS (Elementary Objects of the Nervous System) synaptic modeling platform, which was specifically developed to explore the roles of subsynaptic elements as well as their interactions, and that of synaptic geometry. More specifically, we determined the effects of changing the localization of ionotropic receptors relative to the presynaptic glutamate release site, on synaptic efficacy and its variations following single pulse and paired-pulse stimulation protocols. The results indicate that changes in synaptic geometry do have consequences on synaptic efficacy and its dynamics.
Conflict of interest statement
Figures




References
-
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61(3):421–7. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

