Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice
- PMID: 26480326
- PMCID: PMC4724321
- DOI: 10.1002/cncr.29758
Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice
Abstract
Background: Lynch syndrome confers a hereditary predisposition to colorectal and other cancers. Universal tumor screening (UTS) for Lynch syndrome is recommended by several professional societies, but the implementation can be complex. This article describes the evaluation, process development, and initiation of Lynch syndrome UTS at a tertiary referral cancer center.
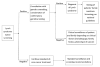
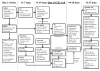
Methods: A multidisciplinary team developed the new process design. Issues in 5 themes were noted: timing, funding, second-opinion patients, result processing, and the role of genetics providers. A committee approach was used to examine each issue for process-improvement development.
Results: The issues related to testing were addressed individually for the successful implementation of UTS at the institutional level. In the conventional-care period, 9 of 30 cases (30%) received Lynch syndrome screening, and 4 cases were referred to medical genetics. During the 6 months following the implementation of UTS, 32 of 44 patients (73%) received Lynch syndrome screening. The 13 unscreened patients all had identified reasons for nonscreening (eg, financial limitations). Ten patients were referred to medical genetics, which identified no new cases of Lynch syndrome, but a low-risk adenomatous polyposis coli (APC) variant was detected in 1 individual.
Conclusions: The implementation of effective Lynch syndrome UTS can feasibly alter practice at the institutional level. This experience with the assessment and management of issues relevant to the successful implementation of a new clinical care paradigm based on emerging technology has implications for the uptake of advances across molecular oncology into clinical practice, and this is highly relevant in the current era of rapidly evolving genomic technology.
Keywords: Lynch syndrome; genetics; genomics; microsatellite instability; molecular oncology.
© 2015 American Cancer Society.
Conflict of interest statement
Figures
References
-
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. - PubMed
-
- Senter L. Genetic testing by cancer site: colon (nonpolyposis syndromes) Cancer J. 2012;18:334–337. - PubMed
-
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. - PubMed
-
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

