Downregulation of hepatic betaine:homocysteine methyltransferase (BHMT) expression in taurine-deficient mice is reversed by taurine supplementation in vivo
- PMID: 26481005
- PMCID: PMC4924537
- DOI: 10.1007/s00726-015-2108-9
Downregulation of hepatic betaine:homocysteine methyltransferase (BHMT) expression in taurine-deficient mice is reversed by taurine supplementation in vivo
Abstract
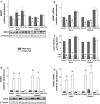
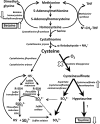
The cysteine dioxygenase (Cdo1)-null and the cysteine sulfinic acid decarboxylase (Csad)-null mouse are not able to synthesize hypotaurine/taurine by the cysteine/cysteine sulfinate pathway and have very low tissue taurine levels. These mice provide excellent models for studying the effects of taurine on biological processes. Using these mouse models, we identified betaine:homocysteine methyltransferase (BHMT) as a protein whose in vivo expression is robustly regulated by taurine. BHMT levels are low in liver of both Cdo1-null and Csad-null mice, but are restored to wild-type levels by dietary taurine supplementation. A lack of BHMT activity was indicated by an increase in the hepatic betaine level. In contrast to observations in liver of Cdo1-null and Csad-null mice, BHMT was not affected by taurine supplementation of primary hepatocytes from these mice. Likewise, CSAD abundance was not affected by taurine supplementation of primary hepatocytes, although it was robustly upregulated in liver of Cdo1-null and Csad-null mice and lowered to wild-type levels by dietary taurine supplementation. The mechanism by which taurine status affects hepatic CSAD and BHMT expression appears to be complex and to require factors outside of hepatocytes. Within the liver, mRNA abundance for both CSAD and BHMT was upregulated in parallel with protein levels, indicating regulation of BHMT and CSAD mRNA synthesis or degradation.
Keywords: Betaine; Betaine:homocysteine methyltransferase; Cysteine dioxygenase; Cysteine sulfinic acid decarboxylase; Hepatocytes; Liver; Taurine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Identification of Taurine-Responsive Genes in Murine Liver Using the Cdo1-Null Mouse Model.Adv Exp Med Biol. 2017;975 Pt 1:475-495. doi: 10.1007/978-94-024-1079-2_38. Adv Exp Med Biol. 2017. PMID: 28849476
-
HNF4α Regulates CSAD to Couple Hepatic Taurine Production to Bile Acid Synthesis in Mice.Gene Expr. 2018 Aug 22;18(3):187-196. doi: 10.3727/105221618X15277685544442. Epub 2018 Jun 5. Gene Expr. 2018. PMID: 29871716 Free PMC article.
-
Taurine alleviates repression of betaine-homocysteine S-methyltransferase and significantly improves the efficacy of long-term betaine treatment in a mouse model of cystathionine β-synthase-deficient homocystinuria.FASEB J. 2019 May;33(5):6339-6353. doi: 10.1096/fj.201802069RR. Epub 2019 Feb 15. FASEB J. 2019. PMID: 30768359
-
Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism?Cell Mol Life Sci. 2006 Dec;63(23):2792-803. doi: 10.1007/s00018-006-6249-6. Cell Mol Life Sci. 2006. PMID: 17086380 Free PMC article. Review.
-
Taurine Regulation of Neuroendocrine Function.Adv Exp Med Biol. 2019;1155:977-985. doi: 10.1007/978-981-13-8023-5_81. Adv Exp Med Biol. 2019. PMID: 31468461 Review.
Cited by
-
Genetic resistance to DEHP-induced transgenerational endocrine disruption.PLoS One. 2019 Jun 10;14(6):e0208371. doi: 10.1371/journal.pone.0208371. eCollection 2019. PLoS One. 2019. PMID: 31181066 Free PMC article.
-
High cysteine diet reduces insulin resistance in SHR-CRP rats.Physiol Res. 2021 Nov 29;70(5):687-700. doi: 10.33549/physiolres.934736. Epub 2021 Sep 10. Physiol Res. 2021. PMID: 34505526 Free PMC article.
-
Betaine Supplementation Into High-Carbohydrate Diets Improves Feed Efficiency and Liver Health of Megalobrama amblycephala by Increasing Taurine Synthesis.Aquac Nutr. 2024 Sep 26;2024:9632883. doi: 10.1155/2024/9632883. eCollection 2024. Aquac Nutr. 2024. PMID: 39555516 Free PMC article.
-
Exploratory Investigation of the Plasma Proteome Associated with the Endotheliopathy of Trauma.Int J Mol Sci. 2022 Jun 1;23(11):6213. doi: 10.3390/ijms23116213. Int J Mol Sci. 2022. PMID: 35682894 Free PMC article.
-
Identification of Taurine-Responsive Genes in Murine Liver Using the Cdo1-Null Mouse Model.Adv Exp Med Biol. 2017;975 Pt 1:475-495. doi: 10.1007/978-94-024-1079-2_38. Adv Exp Med Biol. 2017. PMID: 28849476
References
-
- Agca CA, Tuzcu M, Hayirli A, Sahin K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol. 2014;71:116–121. - PubMed
-
- Bagley PJ, Stipanuk MH. The activities of rat hepatic cysteine dioxygenase and cysteinesulfinate decarboxylase are regulated in a reciprocal manner in response to dietary casein level. J Nutr. 1994;124:2410–2421. - PubMed
-
- Bella DL, Hahn C, Stipanuk MH. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol. 1999a;277(1 Pt 1):E144–E153. - PubMed
-
- Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol. 1999b;276(2 Pt 1):E326–E335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

