Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34(+) cells
- PMID: 26481144
- PMCID: PMC4617445
- DOI: 10.1186/s13287-015-0194-y
Differential ability of MSCs isolated from placenta and cord as feeders for supporting ex vivo expansion of umbilical cord blood derived CD34(+) cells
Abstract
Introduction: Ex vivo expansion of umbilical cord blood (UCB) is attempted to increase cell numbers to overcome the limitation of cell dose. Presently, suspension cultures or feeder mediated co-cultures are performed for expansion of hematopoietic stem cells (HSCs). Mesenchymal stem cells (MSCs) have proved to be efficient feeders for the maintenance of HSCs. Here, we have established MSCs-HSCs co-culture system with MSCs isolated from less invasive and ethically acceptable sources like umbilical cord tissue (C-MSCs) and placenta (P-MSCs). MSCs derived from these tissues are often compared with bone marrow derived MSCs (BM-MSCs) which are considered as a gold standard. However, so far none of the studies have directly compared C-MSCs with P-MSCs as feeders for ex vivo expansion of HSCs. Thus, we for the first time performed a systematic comparison of hematopoietic supportive capability of C and P-MSCs using paired samples.
Methods: UCB-derived CD34(+) cells were isolated and co-cultured on irradiated C and P-MSCs for 10 days. C-MSCs and P-MSCs were isolated from the same donor. The cultures comprised of serum-free medium supplemented with 25 ng/ml each of SCF, TPO, Flt-3 L and IL-6. After 10 days cells were collected and analyzed for phenotype and functionality.
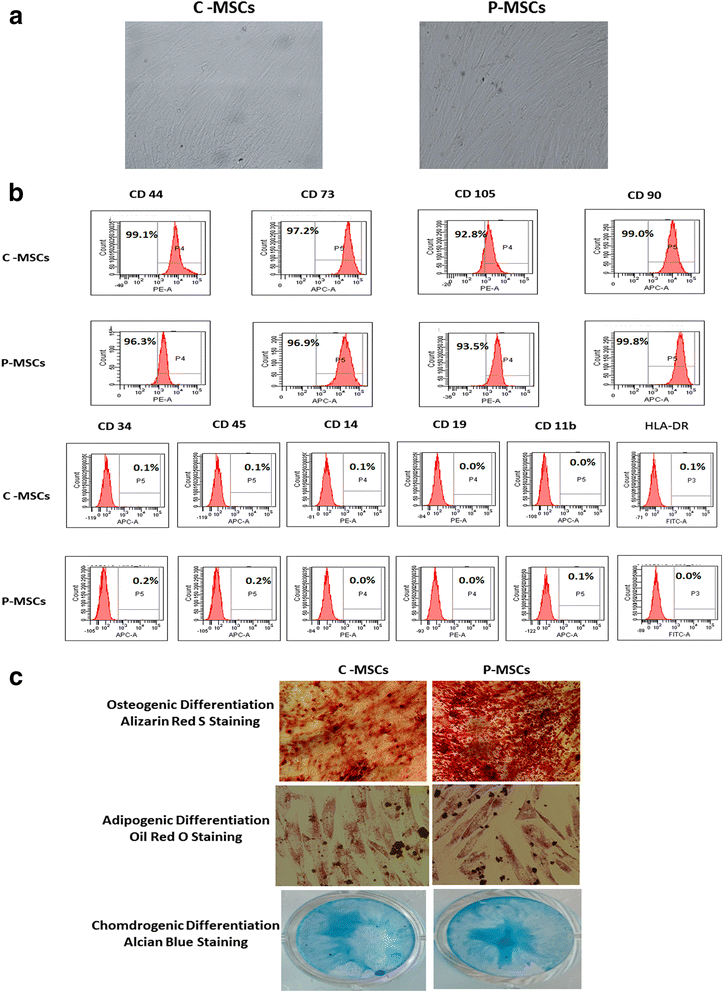
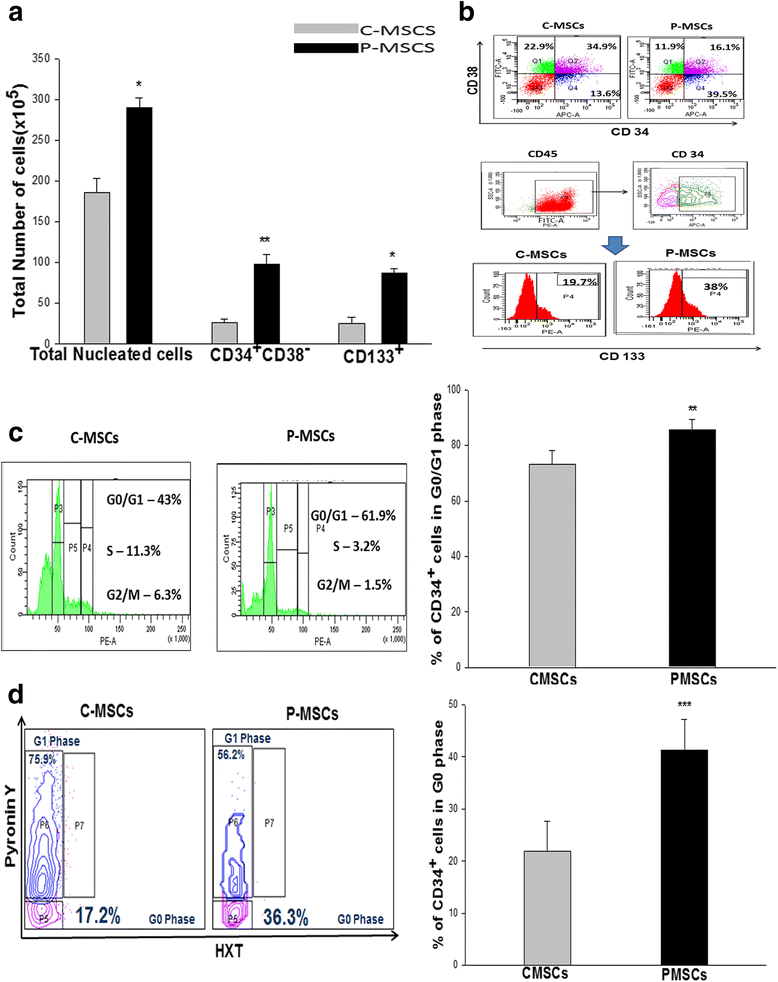
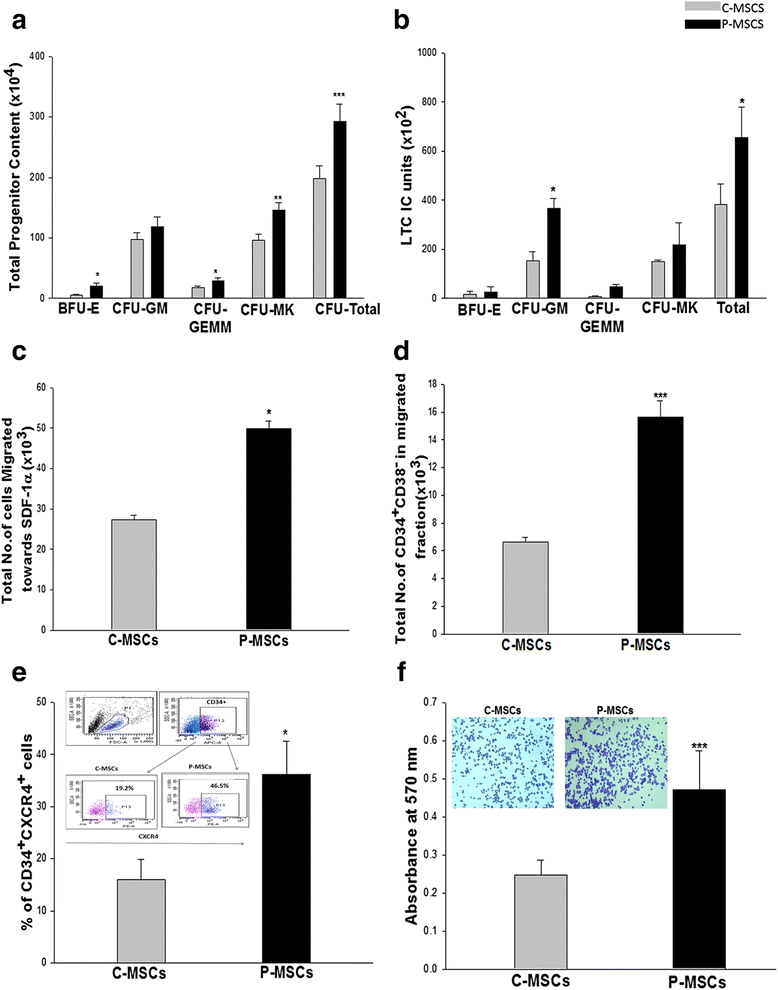
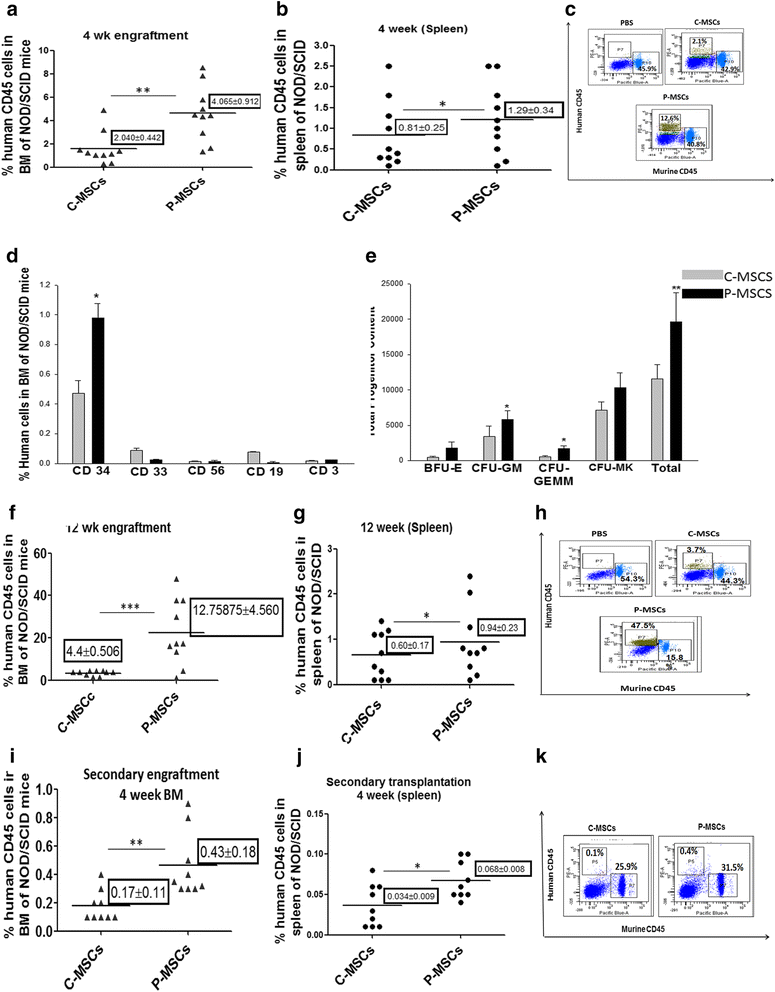
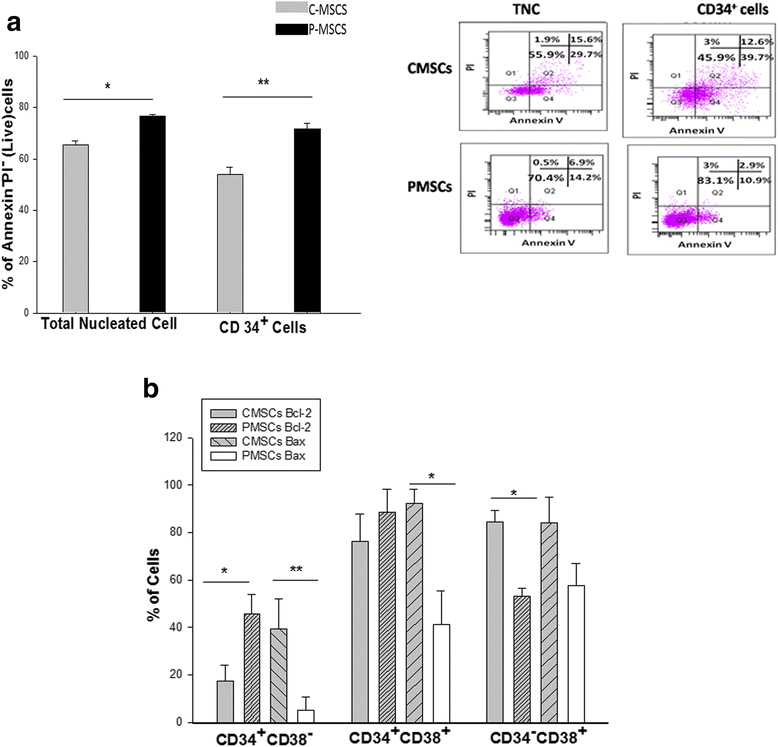
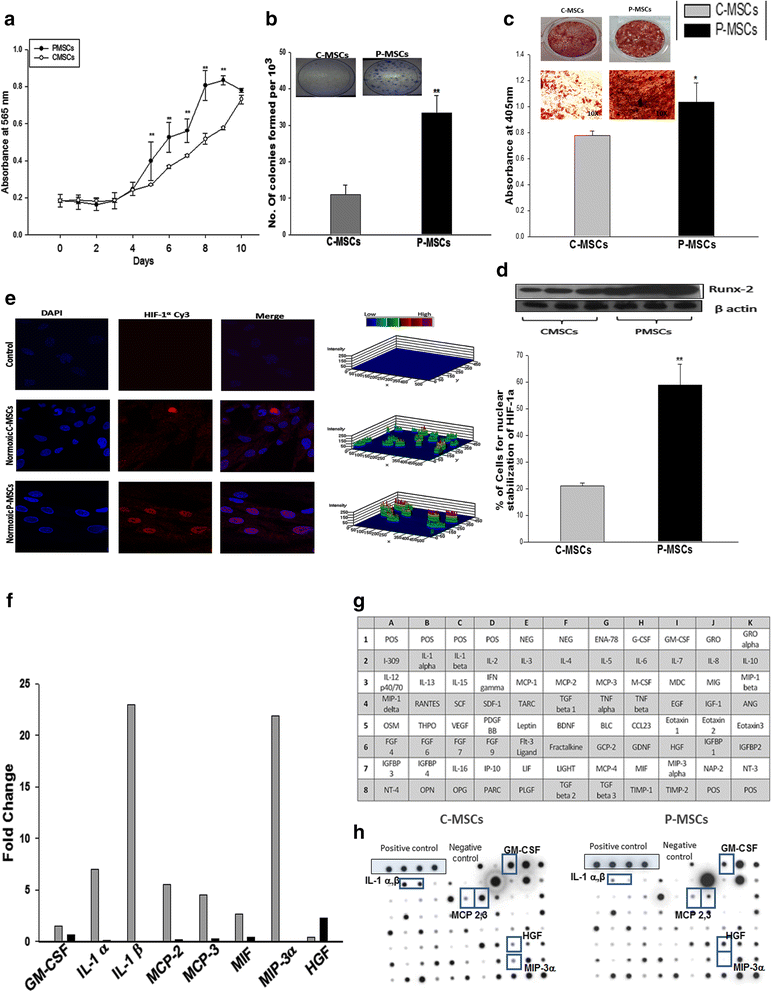
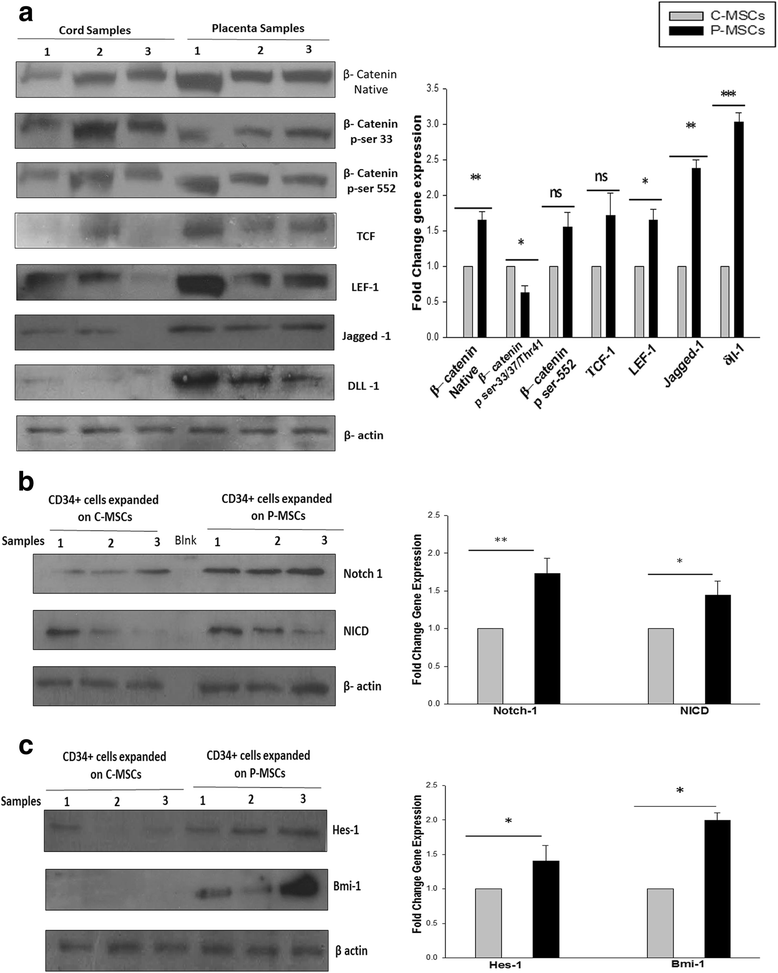
Results: C-MSCs and P-MSCs were found to be morphologically and phenotypically similar but exhibited differential ability to support ex vivo hematopoiesis. Cells expanded on P-MSCs showed higher percentage of primitive cells (CD34(+)CD38(-)), CFU (Colony forming unit) content and LTC-IC (Long term culture initiating cells) ability. CD34(+) cells expanded on P-MSCs also exhibited better in vitro adhesion to fibronectin and migration towards SDF-1α and enhanced NOD/SCID repopulation ability, as compared to those grown on C-MSCs. P-MSCs were found to be closer to BM-MSCs in their ability to expand HSCs. P-MSCs supported expansion of functionally superior HSCs by virtue of reduction in apoptosis of primitive HSCs, higher Wnt and Notch activity, HGF secretion and cell-cell contact. On the other hand, C-MSCs facilitated expansion of progenitors (CD34(+)CD38(+)) and differentiated (CD34(-)CD38(+)) cells by secretion of IL1-α, β, MCP-2, 3 and MIP-3α.
Conclusions: P-MSCs were found to be better feeders for ex vivo maintenance of primitive HSCs with higher engraftment potential than the cells expanded with C-MSCs as feeders.
Figures
Similar articles
-
Wharton's jelly mesenchymal stem cell-based or umbilical vein endothelial cell-based serum-free coculture with cytokines supports the ex vivo expansion/maintenance of cord blood hematopoietic stem/progenitor cells.Stem Cell Res Ther. 2019 Dec 5;10(1):376. doi: 10.1186/s13287-019-1502-8. Stem Cell Res Ther. 2019. PMID: 31806004 Free PMC article.
-
Dynamic cell-cell interactions between cord blood haematopoietic progenitors and the cellular niche are essential for the expansion of CD34+, CD34+CD38- and early lymphoid CD7+ cells.J Tissue Eng Regen Med. 2010 Feb;4(2):149-58. doi: 10.1002/term.226. J Tissue Eng Regen Med. 2010. PMID: 19937912
-
Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood.Cell Transplant. 2007;16(6):579-85. doi: 10.3727/000000007783465073. Cell Transplant. 2007. PMID: 17912949
-
Wharton's Jelly Mesenchymal Stromal Cells as a Feeder Layer for the Ex Vivo Expansion of Hematopoietic Stem and Progenitor Cells: a Review.Stem Cell Rev Rep. 2017 Feb;13(1):35-49. doi: 10.1007/s12015-016-9702-4. Stem Cell Rev Rep. 2017. PMID: 27853939 Review.
-
Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice.Ann N Y Acad Sci. 2001 Jun;938:9-17. doi: 10.1111/j.1749-6632.2001.tb03569.x. Ann N Y Acad Sci. 2001. PMID: 11458530 Review.
Cited by
-
Human PMSCs-derived small extracellular vesicles alleviate neuropathic pain through miR-26a-5p/Wnt5a in SNI mice model.J Neuroinflammation. 2022 Sep 7;19(1):221. doi: 10.1186/s12974-022-02578-9. J Neuroinflammation. 2022. PMID: 36071475 Free PMC article.
-
Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis.Cell Mol Life Sci. 2020 Jan;77(2):253-265. doi: 10.1007/s00018-019-03268-1. Epub 2019 Aug 29. Cell Mol Life Sci. 2020. PMID: 31468060 Free PMC article. Review.
-
Differentiated Cells Derived from Hematopoietic Stem Cells and Their Applications in Translational Medicine.Adv Exp Med Biol. 2021;1347:29-43. doi: 10.1007/5584_2021_644. Adv Exp Med Biol. 2021. PMID: 34114129
-
Wharton's jelly mesenchymal stem cell-based or umbilical vein endothelial cell-based serum-free coculture with cytokines supports the ex vivo expansion/maintenance of cord blood hematopoietic stem/progenitor cells.Stem Cell Res Ther. 2019 Dec 5;10(1):376. doi: 10.1186/s13287-019-1502-8. Stem Cell Res Ther. 2019. PMID: 31806004 Free PMC article.
-
Effect of ectopic high expression of transcription factor OCT4 on the "stemness" characteristics of human bone marrow-derived mesenchymal stromal cells.Stem Cell Res Ther. 2019 Jun 3;10(1):160. doi: 10.1186/s13287-019-1263-4. Stem Cell Res Ther. 2019. PMID: 31159871 Free PMC article.
References
-
- Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant. 2013;6:904–11. doi: 10.1016/j.bbmt.2013.02.008. - DOI - PMC - PubMed
-
- Haspel RL, Ballen KK. Double cord blood transplants: filling a niche? Stem Cell Rev. 2006;2:81–6. - PubMed
-
- Flores–Guzman P, Fernandez–Sanchezv V, Mayani H. Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine. Stem Cells Trans Med. 2013;2:830–8. doi: 10.5966/sctm.2013-0071. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

