Perm1 enhances mitochondrial biogenesis, oxidative capacity, and fatigue resistance in adult skeletal muscle
- PMID: 26481306
- PMCID: PMC4714556
- DOI: 10.1096/fj.15-276360
Perm1 enhances mitochondrial biogenesis, oxidative capacity, and fatigue resistance in adult skeletal muscle
Abstract
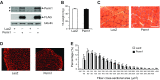
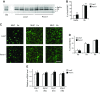
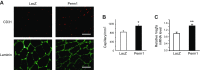
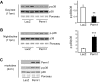
Skeletal muscle mitochondrial content and oxidative capacity are important determinants of muscle function and whole-body health. Mitochondrial content and function are enhanced by endurance exercise and impaired in states or diseases where muscle function is compromised, such as myopathies, muscular dystrophies, neuromuscular diseases, and age-related muscle atrophy. Hence, elucidating the mechanisms that control muscle mitochondrial content and oxidative function can provide new insights into states and diseases that affect muscle health. In past studies, we identified Perm1 (PPARGC1- and ESRR-induced regulator, muscle 1) as a gene induced by endurance exercise in skeletal muscle, and regulating mitochondrial oxidative function in cultured myotubes. The capacity of Perm1 to regulate muscle mitochondrial content and function in vivo is not yet known. In this study, we use adeno-associated viral (AAV) vectors to increase Perm1 expression in skeletal muscles of 4-wk-old mice. Compared to control vector, AAV1-Perm1 leads to significant increases in mitochondrial content and oxidative capacity (by 40-80%). Moreover, AAV1-Perm1-transduced muscles show increased capillary density and resistance to fatigue (by 33 and 31%, respectively), without prominent changes in fiber-type composition. These findings suggest that Perm1 selectively regulates mitochondrial biogenesis and oxidative function, and implicate Perm1 in muscle adaptations that also occur in response to endurance exercise.
Keywords: angiogenesis; endurance exercise responses; oxidative metabolism; skeletal muscle plasticity.
© FASEB.
Figures



References
-
- Hawley J. A., Hargreaves M., Joyner M. J., Zierath J. R. (2014) Integrative biology of exercise. Cell 159, 738–749 - PubMed
-
- Lexell J. (1995) Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 50, 11–16 - PubMed
-
- Kuznetsov A. V., Winkler K., Wiedemann F. R., von Bossanyi P., Dietzmann K., Kunz W. S. (1998) Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol. Cell. Biochem. 183, 87–96 - PubMed
-
- Schiaffino S., Reggiani C. (2011) Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 - PubMed
-
- Russell A. P., Foletta V. C., Snow R. J., Wadley G. D. (2014) Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim. Biophys. Acta 1840, 1276–1284 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

