An agonist antibody that blocks autoimmunity by inducing anti-inflammatory macrophages
- PMID: 26481307
- PMCID: PMC6188224
- DOI: 10.1096/fj.15-281329
An agonist antibody that blocks autoimmunity by inducing anti-inflammatory macrophages
Abstract
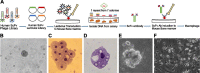
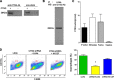
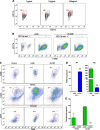
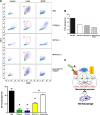
We have devised a method of using intracellular combinatorial libraries to select antibodies that control cell fates. Many agonist antibodies have been selected with this method, and the process appears to be limited only by the availability of a phenotypic selection system. We demonstrate the utility of this approach to discover agonist antibodies that engage an unanticipated target and regulate macrophage polarization by selective induction of anti-inflammatory M2 macrophages. This antibody was used therapeutically to block autoimmunity in a classic mouse model of spontaneous systemic lupus erythematosus.
Keywords: CD14; CTSG; M2 macrophage; cathepsin G.
© FASEB.
Conflict of interest statement
The authors thank Dr. C. Pham (Washington University, St. Louis, MO, USA) and Dr. A. Rao (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA) for knockout mice; and Drs. T. Bartfai, B. Felding, and Y. J. Kang [The Scripps Research Institute (TSRI)] for scientific discussions. Funding was provided by TSRI. K.H. and R.A.L. are the inventors of the LKAb agonist antibody, which has been patented by TSRI. The patent has been licensed to Zebra Technologies. The remaining authors declare no conflicts of interest.
Figures

Similar articles
-
Langerhans Cells Maintain Local Tissue Tolerance in a Model of Systemic Autoimmune Disease.J Immunol. 2015 Jul 15;195(2):464-76. doi: 10.4049/jimmunol.1402735. Epub 2015 Jun 12. J Immunol. 2015. PMID: 26071559 Free PMC article.
-
Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl.Pediatr Dermatol. 2010 Jul-Aug;27(4):373-6. doi: 10.1111/j.1525-1470.2010.01179.x. Pediatr Dermatol. 2010. PMID: 20653856
-
Murine models of cutaneous involvement in lupus erythematosus.Autoimmun Rev. 2009 May;8(6):484-7. doi: 10.1016/j.autrev.2009.02.028. Epub 2009 Feb 23. Autoimmun Rev. 2009. PMID: 19239927 Review.
-
The deposition of anti-DNA IgG contributes to the development of cutaneous lupus erythematosus.Immunol Lett. 2017 Nov;191:1-9. doi: 10.1016/j.imlet.2017.09.003. Epub 2017 Sep 9. Immunol Lett. 2017. PMID: 28899632
-
Cutaneous lupus erythematosus: recent lessons from animal models.Lupus. 2010 Aug;19(9):1029-35. doi: 10.1177/0961203310370045. Lupus. 2010. PMID: 20693196 Review.
Cited by
-
Migration-based selections of antibodies that convert bone marrow into trafficking microglia-like cells that reduce brain amyloid β.Proc Natl Acad Sci U S A. 2018 Jan 16;115(3):E372-E381. doi: 10.1073/pnas.1719259115. Epub 2018 Jan 2. Proc Natl Acad Sci U S A. 2018. PMID: 29295920 Free PMC article.
-
An Antagonist Antibody That Inhibits Cancer Cell Growth In Vitro through RACK1.Pharmaceuticals (Basel). 2024 Sep 30;17(10):1303. doi: 10.3390/ph17101303. Pharmaceuticals (Basel). 2024. PMID: 39458945 Free PMC article.
-
Antibody Libraries as Tools to Discover Functional Antibodies and Receptor Pleiotropism.Int J Mol Sci. 2021 Apr 16;22(8):4123. doi: 10.3390/ijms22084123. Int J Mol Sci. 2021. PMID: 33923551 Free PMC article. Review.
-
The STAT3-IL-10-IL-6 Pathway Is a Novel Regulator of Macrophage Efferocytosis and Phenotypic Conversion in Sterile Liver Injury.J Immunol. 2018 Feb 1;200(3):1169-1187. doi: 10.4049/jimmunol.1701247. Epub 2017 Dec 20. J Immunol. 2018. PMID: 29263216 Free PMC article.
-
Agonist (P1) Antibody Converts Stem Cells into Migrating Beta-Like Cells in Pancreatic Islets.J Microbiol Biotechnol. 2022 Dec 28;32(12):1615-1621. doi: 10.4014/jmb.2209.09031. Epub 2022 Oct 28. J Microbiol Biotechnol. 2022. PMID: 36330755 Free PMC article.
References
-
- Zhang H., Yea K., Xie J., Ruiz D., Wilson I. A., Lerner R. A. (2013) Selecting agonists from single cells infected with combinatorial antibody libraries. Chem. Biol. , 734–741 - PubMed
-
- Zhang H., Xie J., Lerner R. A. (2014) A proximity based general method for identification of ligand and receptor interactions in living cells. Biochem. Biophys. Res. Commun. , 251–255 - PubMed
-
- Xie J., Yea K., Zhang H., Moldt B., He L., Zhu J., Lerner R. A. (2014) Prevention of cell death by antibodies selected from intracellular combinatorial libraries. Chem. Biol. , 274–283 - PubMed
-
- Yea K., Xie J., Zhang H., Zhang W., Lerner R. A. (2015) Selection of multiple agonist antibodies from intracellular combinatorial libraries reveals that cellular receptors are functionally pleiotropic. Curr. Opin. Chem. Biol. , 1–7 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

