TLR9 ligation in pancreatic stellate cells promotes tumorigenesis
- PMID: 26481685
- PMCID: PMC4647258
- DOI: 10.1084/jem.20142162
TLR9 ligation in pancreatic stellate cells promotes tumorigenesis
Abstract
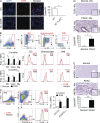
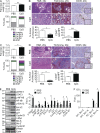
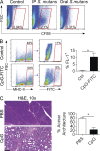
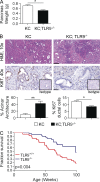
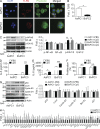
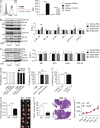
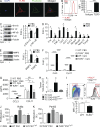
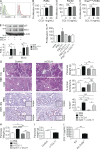
Modulation of Toll-like receptor (TLR) signaling can have protective or protumorigenic effects on oncogenesis depending on the cancer subtype and on specific inflammatory elements within the tumor milieu. We found that TLR9 is widely expressed early during the course of pancreatic transformation and that TLR9 ligands are ubiquitous within the tumor microenvironment. TLR9 ligation markedly accelerates oncogenesis, whereas TLR9 deletion is protective. We show that TLR9 activation has distinct effects on the epithelial, inflammatory, and fibrogenic cellular subsets in pancreatic carcinoma and plays a central role in cross talk between these compartments. Specifically, TLR9 activation can induce proinflammatory signaling in transformed epithelial cells, but does not elicit oncogene expression or cancer cell proliferation. Conversely, TLR9 ligation induces pancreatic stellate cells (PSCs) to become fibrogenic and secrete chemokines that promote epithelial cell proliferation. TLR9-activated PSCs mediate their protumorigenic effects on the epithelial compartment via CCL11. Additionally, TLR9 has immune-suppressive effects in the tumor microenvironment (TME) via induction of regulatory T cell recruitment and myeloid-derived suppressor cell proliferation. Collectively, our work shows that TLR9 has protumorigenic effects in pancreatic carcinoma which are distinct from its influence in extrapancreatic malignancies and from the mechanistic effects of other TLRs on pancreatic oncogenesis.
© 2015 Zambirinis et al.
Figures

Comment in
-
Pancreatic cancer takes its Toll.J Exp Med. 2015 Nov 16;212(12):1988. doi: 10.1084/jem.21212insight1. J Exp Med. 2015. PMID: 26573583 Free PMC article. No abstract available.
References
-
- American Cancer Society 2013. Cancer Facts & Figures 2013. In American Cancer Society, Atlanta.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

