Histidine provides long-term neuroprotection after cerebral ischemia through promoting astrocyte migration
- PMID: 26481857
- PMCID: PMC4611873
- DOI: 10.1038/srep15356
Histidine provides long-term neuroprotection after cerebral ischemia through promoting astrocyte migration
Abstract
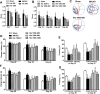
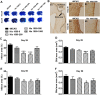
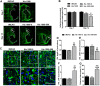
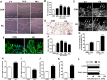
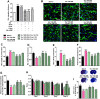
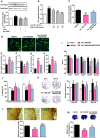
The formation of glial scar impedes the neurogenesis and neural functional recovery following cerebral ischemia. Histamine showed neuroprotection at early stage after cerebral ischemia, however, its long-term effect, especially on glial scar formation, hasn't been characterized. With various administration regimens constructed for histidine, a precursor of histamine, we found that histidine treatment at a high dose at early stage and a low dose at late stage demonstrated the most remarkable long-term neuroprotection with decreased infarct volume and improved neurological function. Notably, this treatment regimen also robustly reduced the glial scar area and facilitated the astrocyte migration towards the infarct core. In wound-healing assay and transwell test, histamine significantly promoted astrocyte migration. H2 receptor antagonists reversed the promotion of astrocyte migration and the neuroprotection provided by histidine. Moreover, histamine upregulated the GTP-bound small GTPase Rac1, while a Rac1 inhibitor, NSC23766, abrogated the neuroprotection of histidine and its promotion of astrocyte migration. Our data indicated that a dose/stage-dependent histidine treatment, mediated by H2 receptor, promoted astrocyte migration towards the infarct core, which benefited long-term post-cerebral ischemia neurological recovery. Therefore, targeting histaminergic system may be an effective therapeutic strategy for long-term cerebral ischemia injury through its actions on astrocytes.
Figures
References
-
- Dirnagl U., Iadecola C. & Moskowitz M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–97 (1999). - PubMed
-
- Lo E. H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 14, 497–500 (2008). - PubMed
-
- Steinberg G. K. et al. Narrow temporal therapeutic window for NMDA antagonist protection against focal cerebral ischaemia. Neurobiol. Dis. 2, 109–18 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

