RETRACTED: Chemotherapy Resistance in Diffuse-Type Gastric Adenocarcinoma Is Mediated by RhoA Activation in Cancer Stem-Like Cells
- PMID: 26482039
- PMCID: PMC4823002
- DOI: 10.1158/1078-0432.CCR-15-1356
RETRACTED: Chemotherapy Resistance in Diffuse-Type Gastric Adenocarcinoma Is Mediated by RhoA Activation in Cancer Stem-Like Cells
Retraction in
-
Retraction: Chemotherapy Resistance in Diffuse-Type Gastric Adenocarcinoma Is Mediated by RhoA Activation in Cancer Stem-Like Cells.Clin Cancer Res. 2024 Oct 15;30(20):4802. doi: 10.1158/1078-0432.CCR-24-2133. Clin Cancer Res. 2024. PMID: 39402968 Free PMC article. No abstract available.
Abstract
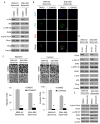
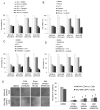
Purpose: The Lauren diffuse type of gastric adenocarcinoma (DGA), as opposed to the intestinal type (IGA), often harbors mutations in RHOA, but little is known about the role of RhoA in DGA.
Experimental design: We examined RhoA activity and RhoA pathway inhibition in DGA cell lines and in two mouse xenograft models. RhoA activity was also assessed in patient tumor samples.
Results: RhoA activity was higher in DGA compared with IGA cell lines and was further increased when grown as spheroids to enrich for cancer stem-like cells (CSCs) or when sorted using the gastric CSC marker CD44. RhoA shRNA or the RhoA inhibitor Rhosin decreased expression of the stem cell transcription factor, Sox2, and decreased spheroid formation by 78% to 81%. DGA spheroid cells had 3- to 5-fold greater migration and invasion than monolayer cells, and this activity was Rho-dependent. Diffuse GA spheroid cells were resistant in a cytotoxicity assay to 5-fluorouracil and cisplatin chemotherapy, and this resistance could be reversed with RhoA pathway inhibition. In two xenograft models, cisplatin inhibited tumor growth by 40% to 50%, RhoA inhibition by 32% to 60%, and the combination by 77% to 83%. In 288 patient tumors, increased RhoA activity correlated with worse overall survival in DGA patients (P = 0.017) but not in IGA patients (P = 0.612).
Conclusions: RhoA signaling promotes CSC phenotypes in DGA cells. Increased RhoA activity is correlated with worse overall survival in DGA patients, and RhoA inhibition can reverse chemotherapy resistance in DGA CSC and in tumor xenografts. Thus, the RhoA pathway is a promising new target in DGA patients.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest: The authors declare no conflicts of interest.
Figures




Similar articles
-
RETRACTED: CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance.Clin Cancer Res. 2014 Aug 1;20(15):3974-88. doi: 10.1158/1078-0432.CCR-14-0011. Epub 2014 Jun 19. Clin Cancer Res. 2014. Retraction in: Clin Cancer Res. 2024 Oct 15;30(20):4803. doi: 10.1158/1078-0432.CCR-24-2134. PMID: 24947926 Free PMC article. Retracted. Clinical Trial.
-
RETRACTED: Role of Rac1 Pathway in Epithelial-to-Mesenchymal Transition and Cancer Stem-like Cell Phenotypes in Gastric Adenocarcinoma.Mol Cancer Res. 2017 Aug;15(8):1106-1116. doi: 10.1158/1541-7786.MCR-17-0053. Epub 2017 May 1. Mol Cancer Res. 2017. Retraction in: Mol Cancer Res. 2024 Nov 1;22(11):1068. doi: 10.1158/1541-7786.MCR-24-0857. PMID: 28461325 Free PMC article. Retracted.
-
RETRACTED: KMT2C Mutations in Diffuse-Type Gastric Adenocarcinoma Promote Epithelial-to-Mesenchymal Transition.Clin Cancer Res. 2018 Dec 15;24(24):6556-6569. doi: 10.1158/1078-0432.CCR-17-1679. Epub 2018 Aug 14. Clin Cancer Res. 2018. Retraction in: Clin Cancer Res. 2024 Oct 15;30(20):4801. doi: 10.1158/1078-0432.CCR-24-2132. PMID: 30108106 Free PMC article. Retracted.
-
Increased RhoA Activity Predicts Worse Overall Survival in Patients Undergoing Surgical Resection for Lauren Diffuse-Type Gastric Adenocarcinoma.Ann Surg Oncol. 2016 Dec;23(13):4238-4246. doi: 10.1245/s10434-016-5357-2. Epub 2016 Jun 30. Ann Surg Oncol. 2016. PMID: 27364501 Free PMC article.
-
RETRACTED: KRAS Activation in Gastric Adenocarcinoma Stimulates Epithelial-to-Mesenchymal Transition to Cancer Stem-Like Cells and Promotes Metastasis.Mol Cancer Res. 2019 Sep;17(9):1945-1957. doi: 10.1158/1541-7786.MCR-19-0077. Epub 2019 Jun 19. Mol Cancer Res. 2019. Retraction in: Mol Cancer Res. 2024 Nov 1;22(11):1066. doi: 10.1158/1541-7786.MCR-24-0858. PMID: 31217166 Free PMC article. Retracted.
Cited by
-
The origin of gastric cancer stem cells and their effects on gastric cancer: Novel therapeutic targets for gastric cancer.Front Oncol. 2022 Sep 15;12:960539. doi: 10.3389/fonc.2022.960539. eCollection 2022. Front Oncol. 2022. PMID: 36185219 Free PMC article. Review.
-
CDK5RAP3 as tumour suppressor negatively regulates self-renewal and invasion and is regulated by ERK1/2 signalling in human gastric cancer.Br J Cancer. 2020 Sep;123(7):1131-1144. doi: 10.1038/s41416-020-0963-y. Epub 2020 Jul 1. Br J Cancer. 2020. PMID: 32606358 Free PMC article.
-
Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice.Nat Commun. 2023 Mar 14;14(1):1402. doi: 10.1038/s41467-023-37142-3. Nat Commun. 2023. PMID: 36918564 Free PMC article.
-
Serine/threonine-protein kinase 24 is an inhibitor of gastric cancer metastasis through suppressing CDH1 gene and enhancing stemness.Am J Cancer Res. 2021 Sep 15;11(9):4277-4293. eCollection 2021. Am J Cancer Res. 2021. PMID: 34659887 Free PMC article.
-
S1PR1 regulates the switch of two angiogenic modes by VE-cadherin phosphorylation in breast cancer.Cell Death Dis. 2019 Feb 27;10(3):200. doi: 10.1038/s41419-019-1411-x. Cell Death Dis. 2019. PMID: 30814488 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9. - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
-
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
-
- Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

