Preclinical Efficacy of the MDM2 Inhibitor RG7112 in MDM2-Amplified and TP53 Wild-type Glioblastomas
- PMID: 26482041
- PMCID: PMC4842012
- DOI: 10.1158/1078-0432.CCR-15-1015
Preclinical Efficacy of the MDM2 Inhibitor RG7112 in MDM2-Amplified and TP53 Wild-type Glioblastomas
Erratum in
-
Correction: Preclinical Efficacy of the MDM2 Inhibitor RG7112 in MDM2-Amplified and TP53 Wild-type Glioblastomas.Clin Cancer Res. 2016 May 1;22(9):2313. doi: 10.1158/1078-0432.CCR-16-0703. Clin Cancer Res. 2016. PMID: 27141010 No abstract available.
Abstract
Purpose: p53 pathway alterations are key molecular events in glioblastoma (GBM). MDM2 inhibitors increase expression and stability of p53 and are presumed to be most efficacious in patients with TP53 wild-type and MDM2-amplified cancers. However, this biomarker hypothesis has not been tested in patients or patient-derived models for GBM.
Experimental design: We performed a preclinical evaluation of RG7112 MDM2 inhibitor, across a panel of 36 patient-derived GBM cell lines (PDCL), each genetically characterized according to their P53 pathway status. We then performed a pharmacokinetic (PK) profiling of RG7112 distribution in mice and evaluated the therapeutic activity of RG7112 in orthotopic and subcutaneous GBM models.
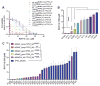
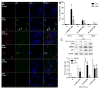
Results: MDM2-amplified PDCLs were 44 times more sensitive than TP53-mutated lines that showed complete resistance at therapeutically attainable concentrations (avg. IC50 of 0.52 μmol/L vs. 21.9 μmol/L). MDM4-amplified PDCLs were highly sensitive but showed intermediate response (avg. IC50 of 1.2 μmol/L), whereas response was heterogeneous in TP53 wild-type PDCLs with normal MDM2/4 levels (avg. IC50 of 7.7 μmol/L). In MDM2-amplified lines, RG7112 restored p53 activity inducing robust p21 expression and apoptosis. PK profiling of RG7112-treated PDCL intracranial xenografts demonstrated that the compound significantly crosses the blood-brain and the blood-tumor barriers. Most importantly, treatment of MDM2-amplified/TP53 wild-type PDCL-derived model (subcutaneous and orthotopic) reduced tumor growth, was cytotoxic, and significantly increased survival.
Conclusions: These data strongly support development of MDM2 inhibitors for clinical testing in MDM2-amplified GBM patients. Moreover, significant efficacy in a subset of non-MDM2-amplified models suggests that additional markers of response to MDM2 inhibitors must be identified.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. - PubMed
-
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22(56):9030–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

