Screen-and-treat program by point-of-care of Atopobium vaginae and Gardnerella vaginalis in preventing preterm birth (AuTop trial): study protocol for a randomized controlled trial
- PMID: 26482128
- PMCID: PMC4616250
- DOI: 10.1186/s13063-015-1000-y
Screen-and-treat program by point-of-care of Atopobium vaginae and Gardnerella vaginalis in preventing preterm birth (AuTop trial): study protocol for a randomized controlled trial
Erratum in
-
Erratum to: 'Screen-and-treat program by point-of-care of Atopobium vaginae and Gardnerella vaginalis in preventing preterm birth (AuTop trial): study protocol for a randomized controlled trial'.Trials. 2016 Feb 12;17:83. doi: 10.1186/s13063-016-1219-2. Trials. 2016. PMID: 26873474 Free PMC article. No abstract available.
Abstract
Background: International recommendations in favor of screening for vaginal infection in pregnancy are based on heterogeneous criteria. In most developed countries, the diagnosis of bacterial vaginosis is only recommended for women with high-risk of preterm birth. The Nugent score is currently used, but molecular quantification tools have recently been reported with a high sensitivity and specificity. Their value for reducing preterm birth rates and related complications remains unexplored. This trial was designed to assess the cost-effectiveness of a systematic screen-and-treat program based on a point-of-care technique for rapid molecular diagnosis, immediately followed by an appropriate antibiotic treatment, to detect the presence of abnormal vaginal flora (specifically, Atopobium vaginae and Gardnerella vaginalis) before 20 weeks of gestation in pregnant women in France. We hypothesized that this program would translate into significant reductions in both the rate of preterm births and the medical costs associated with preterm birth.
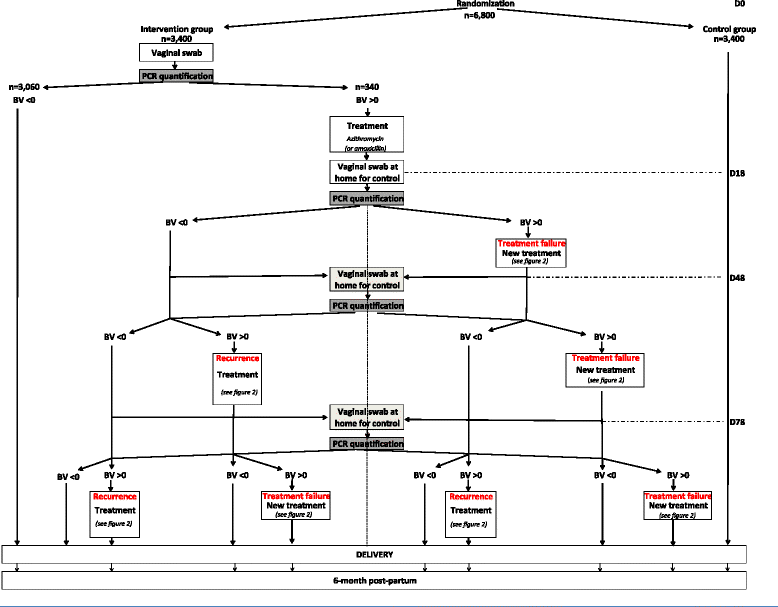
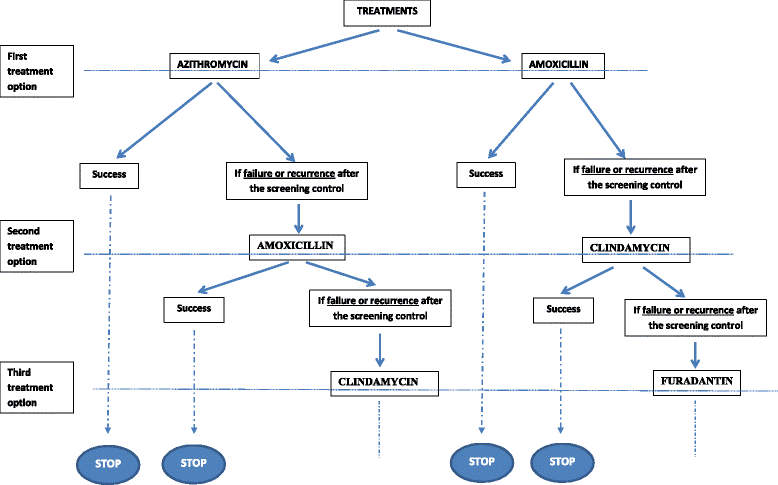
Methods/design: A multicenter, open-label randomized controlled trial (RCT) will be conducted in which 20 French obstetrics and gynecology centers will recruit eligible pregnant women at less than 20 weeks gestation with singleton pregnancy and with a low-risk factor for preterm birth. Interventions will include a) an experimental group that will receive a systematic rapid screen-and-treat program from a point-of-care analysis using a molecular quantification method and b) a control group that will receive usual care management. Randomization will be in a 1:1 allocation ratio. The primary endpoint that will be assessed over a period of 12 months will be the incremental cost-effectiveness ratio (ICER) expressed as cost per avoided preterm birth before 37 weeks. Secondary endpoints will include ICER per avoided preterm birth before 24, 28 and 32 weeks, obstetrical outcomes, neonatal outcomes, rates of treatment failure and recurrence episodes for positive women. Uncertainty surrounding these estimates will be addressed using nonparametric bootstrapping and represented using cost-effectiveness acceptability curves. A total of 6,800 pregnant women will be included.
Discussion: This appropriate randomized controlled design will provide insight into the cost-effectiveness and therefore the potential cost savings of a rapid screen-and-treat strategy for molecular abnormal vaginal flora in pregnant women. National and international recommendations could be updated based on the findings of this study.
Trial registration: ClinicalTrials.gov: NCT02288832 (registration date: 30 October 2014); Eudract: 2014-001559-22.
Figures
References
-
- Blondel B, Lelong N, Kermarrec M, Goffinet F. National Coordination Group of the National Perinatal S. Trends in perinatal health in France from 1995 to 2010. Results from the French National Perinatal Surveys. J Gynecol Obstet Biol Reprod (Paris) 2012;41:e1–e15. doi: 10.1016/j.jgyn.2012.04.014. - DOI - PubMed
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical