Structural analysis of hierarchically organized zeolites
- PMID: 26482337
- PMCID: PMC4667694
- DOI: 10.1038/ncomms9633
Structural analysis of hierarchically organized zeolites
Abstract
Advances in materials synthesis bring about many opportunities for technological applications, but are often accompanied by unprecedented complexity. This is clearly illustrated by the case of hierarchically organized zeolite catalysts, a class of crystalline microporous solids that has been revolutionized by the engineering of multilevel pore architectures, which combine unique chemical functionality with efficient molecular transport. Three key attributes, the crystal, the pore and the active site structure, can be expected to dominate the design process. This review examines the adequacy of the palette of techniques applied to characterize these distinguishing features and their catalytic impact.
Figures

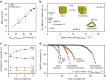
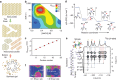
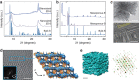
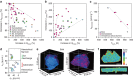

References
-
- Lakes R. Materials with structural hierarchy. Nature 361, 511–515 (1993) .
-
- Brinker C. J. Porous inorganic materials. Curr. Opin. Solid State Mater. Sci. 1, 798–805 (1996) .
-
- Li Y., Fu Z.-Y. & Su B.-L. Hierarchically-structured porous materials for energy conversion and storage. Adv. Funct. Mater. 22, 4634–4667 (2012) .
-
- Gabardo C. M., Zhu Y., Soleymani L. & Moran-Mirabal J. M. Bench-top fabrication of hierarchically structured high-surface-area electrodes. Adv. Funct. Mater. 23, 3030–3039 (2013) .
-
- Hartmann M. Hierarchical zeolites: a proven strategy to combine shape selectivity with efficient mass transport. Angew. Chem. Int. Ed. 43, 5880–5882 (2004) . - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

