Milnacipran for pain in fibromyalgia in adults
- PMID: 26482422
- PMCID: PMC6481368
- DOI: 10.1002/14651858.CD008244.pub3
Milnacipran for pain in fibromyalgia in adults
Abstract
Background: This is an updated version of the original Cochrane review published in Issue 3, 2012. That review considered both fibromyalgia and neuropathic pain, but the efficacy of milnacipran for neuropathic pain is now dealt with in a separate review.Milnacipran is a serotonin-norepinephrine (noradrenaline) reuptake inhibitor (SNRI) that is licensed for the treatment of fibromyalgia in some countries, including Canada, Russia, and the United States.
Objectives: To assess the analgesic efficacy of milnacipran for pain in fibromyalgia in adults and the adverse events associated with its use in clinical trials.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE to 18 May 2015, together with reference lists of retrieved papers and reviews, and two clinical trial registries. For the earlier review, we also contacted the manufacturer.
Selection criteria: We included randomised, double-blind studies of eight weeks' duration or longer, comparing milnacipran with placebo or another active treatment in fibromyalgia in adults.
Data collection and analysis: We extracted efficacy and adverse event data, and two review authors examined issues of study quality independently.
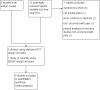
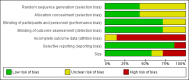
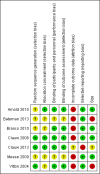
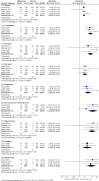
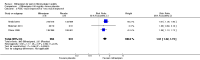
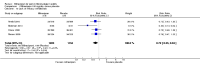
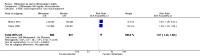
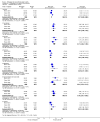
Main results: We identified one new study with 100 participants for the pooled analysis. We identified two additional reports of a study using an enriched enrolment randomised withdrawal (EERW) design that included participants from earlier randomised controlled trials and an open-label study. Because this study used the same participants already included in our main analysis, and a different design, we dealt with it separately.The main analysis included six studies (five from the earlier review; 4238 participants in total), all of which were placebo-controlled, and used titration to a target dose of milnacipran 100 or 200 mg, with assessment after 8 to 24 weeks of stable treatment. There were no studies with active comparators. Study quality was generally good, although the imputation method used in analyses of the primary outcomes could overestimate treatment effect.Both doses of milnacipran provided moderate levels of pain relief (at least 30% pain intensity reduction) to about 40% of participants treated, compared to 30% with placebo, giving a number needed to treat for an additional beneficial outcome (NNT) of 6 to 10 (high quality evidence). Using a stricter definition for responder and a more conservative method of analysis gave lower levels of response (while maintaining a 10% difference between milnacipran and placebo) and increased the NNT to 11 (high quality evidence). One EERW study was broadly supportive.Adverse events were common in both milnacipran (86%) and placebo (78%) groups (high quality evidence), but serious adverse events did not differ between groups (less than 2%) (low quality evidence). Nausea, constipation, and headache were the most common events showing the greatest difference between groups (number needed to treat for an additional harmful outcome (NNH) of 5.7 for nausea, 13 for constipation, and 29 for headache) (moderate quality evidence).Withdrawals for any reason were more common with milnacipran than placebo, and more common with 200 mg (NNH 9) than 100 mg (NNH 23), compared with placebo. This was largely driven by adverse event withdrawals, where the NNH compared with placebo was 14 for 100 mg and 7.0 for 200 mg (high quality evidence). Withdrawals due to lack of efficacy were less common with milnacipran than placebo but did not differ between doses (number needed to treat to prevent an additional unwanted outcome (NNTp) of 41) (moderate quality evidence).
Authors' conclusions: The evidence available indicates that milnacipran 100 mg or 200 mg is effective for a minority in the treatment of pain due to fibromyalgia, providing moderate levels of pain relief (at least 30%) to about 40% of participants, compared with about 30% with placebo. There were insufficient data to assess substantial levels of pain relief (at least 50%), and the use of last observation carried forward imputation may overestimate drug efficacy. Using stricter criteria for 'responder' and a more conservative method of analysis gave lower response rates (about 26% with milnacipran versus 17% with placebo). Milnacipran was associated with increased adverse events and adverse event withdrawals, which were significantly greater for the higher dose.
Conflict of interest statement
MC has no conflicts relating to this review or any similar product.
SD has no conflicts relating to this review or any similar product.
TP has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
We are funded by the National Institute for Health Research (NIHR) for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Figures


















Update of
-
Milnacipran for neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. 2012 Mar 14;3(3):CD008244. doi: 10.1002/14651858.CD008244.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Oct 20;(10):CD008244. doi: 10.1002/14651858.CD008244.pub3. PMID: 22419330 Free PMC article. Updated.
References
References to studies included in this review
Arnold 2010 {published data only}
Bateman 2013 {published data only}
-
- Study of milnacipran in patients with inadequate response to duloxetine for the treatment of fibromyalgia, 2011. www.clinicaltrials.gov/ct2/show/study/NCT01077375 (accessed 22 September 2015). [CTG: NCT01077375]
Branco 2010 {published data only}
Clauw 2008 {published data only}
-
- Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15‐week, multicenter, randomized, double‐blind, placebo‐controlled, multiple‐dose clinical trial. Clinical Therapeutics 2008;30(11):1988‐2004. [DOI: 10.1016/j.clinthera.2008.11.009] - DOI - PubMed
Clauw 2013 {published data only}
-
- Mease PJ, Clauw DJ, Trugman JM, Palmer RH, Wang Y. Efficacy of long‐term milnacipran treatment in patients meeting different thresholds of clinically relevant pain relief: subgroup analysis of a randomized, double‐blind, placebo‐controlled withdrawal study. Journal of Pain Research 2014;7:679‐87. [DOI: 10.2147/JPR.S70200] - DOI - PMC - PubMed
Mease 2009 {published data only}
Vitton 2004 {published data only}
-
- Gendreau RM, Thorn MD, Gendreau JF, Kranzler JD, Ribeiro S, Gracely RH, et al. Efficacy of milnacipran in patients with fibromyalgia. Journal of Rheumatology 2005;32:1975‐85. [PUBMED: 16206355] - PubMed
References to studies excluded from this review
Ang 2013 {published data only}
Arnold 2012 {published data only}
Branco 2011 {published data only}
-
- Branco JC, Cherin P, Montagne A, Bouroubi A, on behalf of the Multinational Coordinator Study Group. Longterm therapeutic response to milnacipran treatment for fibromyalgia. A European 1‐year extension study following a 3‐month study. Journal of Rheumatology 2011;38(7):1403‐12. [DOI: 10.3899/jrheum.101025] - DOI - PubMed
Goldenberg 2010 {published data only}
-
- Goldenberg DL, Clauw DJ, Palmer RH, Mease P, Chen W, Gendreau RM. Durability of therapeutic response to milnacipran treatment for fibromyalgia. Results of a randomized, double‐blind, monotherapy 6‐month extension study. Pain Medicine 2010;11(2):180‐94. [DOI: 10.1111/j.1526-4637.2009.00755.x] - DOI - PubMed
Häuser 2014 {published data only}
-
- Häuser W, Sarzi‐Puttini P, Töle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta‐analysis. Clinical and Experimental Rheumatology 2014;30(6 (Suppl 74)):78‐87. [PUBMED: 23137770] - PubMed
Kim 2013 {published data only}
-
- Kim JL, Rele S, Marks DM, Masand PS, Yerramsetty P, Millet RA, et al. Effects of milnacipran on neurocognition, pain, and fatigue in fibromyalgia: a 13‐week, randomized, placebo‐controlled, crossover trial. Primary Care Companion for CNS Disorders 2013;15(6):PCC.13m01555. [DOI: 10.4088/PCC.13m01555] - DOI - PMC - PubMed
Matthey 2013 {published data only}
-
- Matthey A, Cedraschi C, Piguet V, Besson M, Chabert J, Daali Y, et al. Dual reuptake inhibitor milnacipran and spinal pain pathways in fibromyalgia patients: a randomized, double‐blind, placebo‐controlled trial. Pain Physician 2013;16(5):E553‐62. [PUBMED: 24077206] - PubMed
Mease 2014 {published data only}
NCT00797797 {unpublished data only}
-
- A multicenter, randomized, open‐label, controlled study to evaluate the safety, tolerability, and efficacy of milnacipran when added to pregabalin in the treatment of fibromyalgia, 2011. clinicaltrials.gov/ct2/show/NCT00797797 (accessed 12 August 2011).
Spera 2012 {published data only}
-
- Spera A, Clauw DJ, Arnold LM, Ma Y. Long‐term efficacy and tolerability of milnacipran for the management of fibromyalgia: results from an open‐label, flexible‐dosing study followed by a randomized, double‐blind, placebo‐controlled discontinuation study. Journal of General Internal Medicine. Conference: 35th Annual Meeting of the Society of General Internal Medicine, SGIM. 2012.
Additional references
Arnold 2013
Bernstein 2013
Choy 2011
Dechartres 2013
Derry 2015
Dworkin 2008
Higgins 2011
-
- Higgins JPT, Green S. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2010
Häuser 2011
Häuser 2013
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] - PubMed
Kalso 2013
Kjaergard 2001
Koroschetz 2011
Kyle 2010
L'Abbé 1987
Lunn 2014
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain‐establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] - DOI - PubMed
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - PMC - PubMed
Moore 2010c
Moore 2010d
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011b
Moore 2012a
Moore 2012b
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Nüesch 2010
Ormseth 2010
PaPaS 2012
-
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 11 August 2015).
Queiroz 2013
Recla 2010
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roskell 2011
Saarto 2007
Schmidt‐Wilcke 2014
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Straube 2011
Sultan 2008
Suzuki 2004
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Wiffen 2013
Wolfe 1990
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33:160‐72. [PUBMED: 2306288] - PubMed
Wolfe 2010
Wolfe 2013a
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

