Specific degradation of phosphatidylglycerol is necessary for proper mitochondrial morphology and function
- PMID: 26482708
- PMCID: PMC4690203
- DOI: 10.1016/j.bbabio.2015.10.004
Specific degradation of phosphatidylglycerol is necessary for proper mitochondrial morphology and function
Abstract
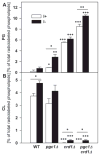
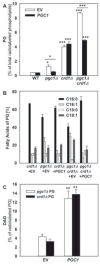
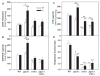
In yeast, phosphatidylglycerol (PG) is a minor phospholipid under standard conditions; it can be utilized for cardiolipin (CL) biosynthesis by CL synthase, Crd1p, or alternatively degraded by the phospholipase Pgc1p. The Saccharomyces cerevisiae deletion mutants crd1Δ and pgc1Δ both accumulate PG. Based on analyses of the phospholipid content of pgc1Δ and crd1Δ yeast, we revealed that in yeast mitochondria, two separate pools of PG are present, which differ in their fatty acid composition and accessibility for Pgc1p-catalyzed degradation. In contrast to CL-deficient crd1Δ yeast, the pgc1Δ mutant contains normal levels of CL. This makes the pgc1Δ strain a suitable model to study the effect of accumulation of PG per se. Using fluorescence microscopy, we show that accumulation of PG with normal levels of CL resulted in increased fragmentation of mitochondria, while in the absence of CL, accumulation of PG led to the formation of large mitochondrial sheets. We also show that pgc1Δ mitochondria exhibited increased respiration rates due to increased activity of cytochrome c oxidase. Taken together, our results indicate that not only a lack of anionic phospholipids, but also excess PG, or unbalanced ratios of anionic phospholipids in mitochondrial membranes, have harmful consequences on mitochondrial morphology and function.
Keywords: Mitochondria; Morphology; Phosphatidylglycerol; Respiration; Yeast.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Two Different Phospholipases C, Isc1 and Pgc1, Cooperate To Regulate Mitochondrial Function.Microbiol Spectr. 2022 Dec 21;10(6):e0248922. doi: 10.1128/spectrum.02489-22. Epub 2022 Nov 15. Microbiol Spectr. 2022. PMID: 36377885 Free PMC article.
-
Yeast Pgc1p (YPL206c) controls the amount of phosphatidylglycerol via a phospholipase C-type degradation mechanism.J Biol Chem. 2008 Jun 20;283(25):17107-15. doi: 10.1074/jbc.M800868200. Epub 2008 Apr 23. J Biol Chem. 2008. PMID: 18434318 Free PMC article.
-
The phosphatidylglycerol/cardiolipin biosynthetic pathway is required for the activation of inositol phosphosphingolipid phospholipase C, Isc1p, during growth of Saccharomyces cerevisiae.J Biol Chem. 2005 Feb 25;280(8):7170-7. doi: 10.1074/jbc.M411058200. Epub 2004 Dec 15. J Biol Chem. 2005. PMID: 15611094
-
Origin and diversification of the cardiolipin biosynthetic pathway in the Eukarya domain.Biochem Soc Trans. 2020 Jun 30;48(3):1035-1046. doi: 10.1042/BST20190967. Biochem Soc Trans. 2020. PMID: 32490527 Review.
-
Cardiolipin synthase from yeast.Biochim Biophys Acta. 1997 Sep 4;1348(1-2):201-6. doi: 10.1016/s0005-2760(97)00117-3. Biochim Biophys Acta. 1997. PMID: 9370334 Review.
Cited by
-
A Saccharomyces cerevisiae knockout screen for genes critical for growth under sulfur- and nitrogen-limited conditions reveals intracellular sorting via vesicular transport systems.G3 (Bethesda). 2025 Jul 9;15(7):jkaf074. doi: 10.1093/g3journal/jkaf074. G3 (Bethesda). 2025. PMID: 40205732 Free PMC article.
-
Two Different Phospholipases C, Isc1 and Pgc1, Cooperate To Regulate Mitochondrial Function.Microbiol Spectr. 2022 Dec 21;10(6):e0248922. doi: 10.1128/spectrum.02489-22. Epub 2022 Nov 15. Microbiol Spectr. 2022. PMID: 36377885 Free PMC article.
-
Misregulation of a DDHD Domain-containing Lipase Causes Mitochondrial Dysfunction in Yeast.J Biol Chem. 2016 Aug 26;291(35):18562-81. doi: 10.1074/jbc.M116.733378. Epub 2016 Jul 8. J Biol Chem. 2016. PMID: 27402848 Free PMC article.
-
The transfer of specific mitochondrial lipids and proteins to lipid droplets contributes to proteostasis upon stress and aging in the eukaryotic model system Saccharomyces cerevisiae.Geroscience. 2020 Feb;42(1):19-38. doi: 10.1007/s11357-019-00103-0. Epub 2019 Nov 1. Geroscience. 2020. PMID: 31676965 Free PMC article.
-
Prognostic biomarkers of cervical squamous cell carcinoma identified via plasma metabolomics.Medicine (Baltimore). 2019 Jun;98(26):e16192. doi: 10.1097/MD.0000000000016192. Medicine (Baltimore). 2019. PMID: 31261558 Free PMC article.
References
-
- Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;946:227–234. - PubMed
-
- Simbeni R, Pon L, Zinser E, Paltauf F, Daum G. Mitochondrial membrane contact sites of yeast. Characterization of lipid components and possible involvement in intramitochondrial translocation of phospholipids. J Biol Chem. 1991;266:10047–10049. - PubMed
-
- Jiang F, Rizavi HS, Greenberg ML. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol Microbiol. 1997;26:481–491. - PubMed
-
- Tuller G, Hrastnik C, Achleitner G, Schiefthaler U, Klein F, Daum G. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS Lett. 1998;421:15–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

