Learning by subtraction: Hippocampal activity and effects of ethanol during the acquisition and performance of response sequences
- PMID: 26482846
- PMCID: PMC4837033
- DOI: 10.1002/hipo.22545
Learning by subtraction: Hippocampal activity and effects of ethanol during the acquisition and performance of response sequences
Abstract
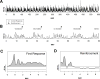
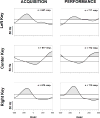
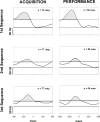
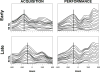
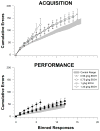
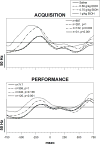
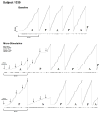
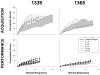
Learning is believed to be reflected in the activity of the hippocampus. However, neural correlates of learning have been difficult to characterize because hippocampal activity is integrated with ongoing behavior. To address this issue, male rats (n = 5) implanted with electrodes (n = 14) in the CA1 subfield responded during two tasks within a single test session. In one task, subjects acquired a new 3-response sequence (acquisition), whereas in the other task, subjects completed a well-rehearsed 3-response sequence (performance). Both tasks though could be completed using an identical response topography and used the same sensory stimuli and schedule of reinforcement. More important, comparing neural patterns during sequence acquisition to those during sequence performance allows for a subtractive approach whereby activity associated with learning could potentially be dissociated from the activity associated with ongoing behavior. At sites where CA1 activity was closely associated with behavior, the patterns of activity were differentially modulated by key position and the serial position of a response within the schedule of reinforcement. Temporal shifts between peak activity and responding on particular keys also occurred during sequence acquisition, but not during sequence performance. Ethanol disrupted CA1 activity while producing rate-decreasing effects in both tasks and error-increasing effects that were more selective for sequence acquisition than sequence performance. Ethanol also produced alterations in the magnitude of modulations and temporal pattern of CA1 activity, although these effects were not selective for sequence acquisition. Similar to ethanol, hippocampal micro-stimulation decreased response rate in both tasks and selectively increased the percentage of errors during sequence acquisition, and provided a more direct demonstration of hippocampal involvement during sequence acquisition. Together, these results strongly support the notion that ethanol disrupts sequence acquisition by disrupting hippocampal activity and that the hippocampus is necessary for the conditioned associations required for sequence acquisition.
Keywords: CA1; micro-stimulation; multi-unit activity; rat; repeated acquisition.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Relative potency and effectiveness of flunitrazepam, ethanol, and beta-CCE for disrupting the acquisition and retention of response sequences in rats.Behav Pharmacol. 2009 Feb;20(1):33-44. doi: 10.1097/FBP.0b013e3283242f2d. Behav Pharmacol. 2009. PMID: 19179849 Free PMC article.
-
Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region.Brain Res. 2005 Jun 28;1048(1-2):69-79. doi: 10.1016/j.brainres.2005.04.041. Brain Res. 2005. PMID: 15919065
-
Effects of neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one on ethanol-mediated paired-pulse depression of population spikes in the CA1 region of rat hippocampal slices.Neurosci Lett. 2006 Feb 6;394(1):28-32. doi: 10.1016/j.neulet.2005.09.062. Epub 2005 Dec 27. Neurosci Lett. 2006. PMID: 16377087
-
Learning alcohol tolerance: the contribution of response expectancies.Psychopharmacology (Berl). 1989;98(3):289-96. doi: 10.1007/BF00451677. Psychopharmacology (Berl). 1989. PMID: 2501809 Review.
-
Repeated acquisition and the assessment of centrally acting compounds.Brain Res Cogn Brain Res. 1996 Jun;3(3-4):183-91. doi: 10.1016/0926-6410(96)00005-5. Brain Res Cogn Brain Res. 1996. PMID: 8806021 Review.
Cited by
-
Understanding ethanol's acute effects on medial prefrontal cortex neural activity using state-space approaches.Neuropharmacology. 2021 Oct 15;198:108780. doi: 10.1016/j.neuropharm.2021.108780. Epub 2021 Sep 1. Neuropharmacology. 2021. PMID: 34480911 Free PMC article. Review.
-
Acute Low Alcohol Disrupts Hippocampus-Striatum Neural Correlate of Learning Strategy by Inhibition of PKA/CREB Pathway in Rats.Front Pharmacol. 2018 Dec 6;9:1439. doi: 10.3389/fphar.2018.01439. eCollection 2018. Front Pharmacol. 2018. PMID: 30574089 Free PMC article.
-
Ethanol Alters Variability, But Not Rate, of Firing in Medial Prefrontal Cortex Neurons of Awake-Behaving Rats.Alcohol Clin Exp Res. 2020 Nov;44(11):2225-2238. doi: 10.1111/acer.14463. Epub 2020 Oct 16. Alcohol Clin Exp Res. 2020. PMID: 32966634 Free PMC article.
-
Chronic Binge Alcohol Administration Dysregulates Hippocampal Genes Involved in Immunity and Neurogenesis in Simian Immunodeficiency Virus-Infected Macaques.Biomolecules. 2016 Nov 9;6(4):43. doi: 10.3390/biom6040043. Biomolecules. 2016. PMID: 27834864 Free PMC article.
References
-
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Research. 1992;598:173–184. - PubMed
-
- Berger TW, Berry SD, Thompson RF. Role of the hippocampus in classical conditioning of aversive and appetitive behaviors. In: Isaacson RL, Pribram KI-I, editors. The Hippocampus. New York: Plenum Press; 1986. pp. 203–239.
-
- Boren JJ. Repeated acquisition of new behavioral chains. American Psychologist. 1963;17:421. (Abstract)
-
- Bostock E, Muller RU, Kubie JL. Experience dependent modifications of hippocampal place cell firing. Hippocampus. 1991;2:193–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

