Zeaxanthin and α-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells
- PMID: 26482868
- PMCID: PMC4847939
- DOI: 10.1016/j.freeradbiomed.2015.10.411
Zeaxanthin and α-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells
Abstract
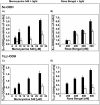
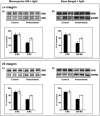
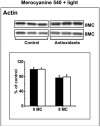
Zeaxanthin and α-tocopherol have been previously shown to efficiently protect liposomal membrane lipids against photosensitized peroxidation, and to protect cultured RPE cells against photodynamic killing. Here the protective action of combined zeaxanthin and α-tocopherol was analyzed in ARPE-19 cells subjected to photodynamic (PD) stress mediated by rose Bengal (RB) or merocyanine-540 (MC-540) at sub-lethal levels. Stress-induced cytotoxicity was analyzed by the MTT assay. The peroxidation of membrane lipids was determined by HPLC-EC (Hg) measurements of cholesterol hydroperoxides using cholesterol as a mechanistic reporter molecule. The specific phagocytosis of FITC-labeled photoreceptor outer segments (POS) isolated from bovine retinas was measured by flow cytometry, and the levels of phagocytosis receptor proteins αv integrin subunit, β5 integrin subunit and MerTK were quantified by Western blot analysis. Cytotoxicity measures confirmed that PD stress levels used for phagocytosis analysis were sub-lethal and that antioxidant supplementation protected against higher, lethal PD doses. Sub-lethal PD stress mediated by both photosensitizers induced the accumulation of 5α-OOH and 7α/β-OOH cholesterol hydroperoxides and the addition of the antioxidants substantially inhibited their accumulation. Antioxidant delivery prior to PD stress also reduced the inhibitory effect of stress on POS phagocytosis and partially reduced the stress-induced diminution of phagocytosis receptor proteins. The use of a novel model system where oxidative stress was induced at sub-lethal levels enable observations that would not be detectable using lethal stress models. Moreover, novel observations about the protective effects of zeaxanthin and α-tocopherol on photodynamic damage to ARPE-19 cell membranes and against reductions in the abundance of receptor proteins involved in POS phagocytosis, a process essential for photoreceptor survival, supports the importance of the antioxidants in protecting of the retina against photooxidative injury.
Keywords: ARPE-19 cells; Antioxidants; Cholesterol hydroperoxides; Lipid peroxidation; Phagocytosis; Phagocytosis receptors MerTK and αvβ5 integrin; Photoreceptor outer segments; Zeaxanthin; α-Tocopherol.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


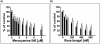
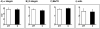

Similar articles
-
Photosensitized oxidative stress to ARPE-19 cells decreases protein receptors that mediate photoreceptor outer segment phagocytosis.Invest Ophthalmol Vis Sci. 2013 Mar 28;54(3):2276-87. doi: 10.1167/iovs.12-11154. Invest Ophthalmol Vis Sci. 2013. PMID: 23449722 Free PMC article.
-
Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids.Free Radic Biol Med. 2004 May 1;36(9):1094-101. doi: 10.1016/j.freeradbiomed.2004.02.005. Free Radic Biol Med. 2004. PMID: 15082063
-
Lipofuscin-mediated photic stress inhibits phagocytic activity of ARPE-19 cells; effect of donors' age and antioxidants.Free Radic Res. 2017 Oct;51(9-10):799-811. doi: 10.1080/10715762.2017.1380307. Epub 2017 Oct 3. Free Radic Res. 2017. PMID: 28969450
-
Nitric Oxide Inhibition of Chain Lipid Peroxidation Initiated by Photodynamic Action in Membrane Environments.Cell Biochem Biophys. 2020 Jun;78(2):149-156. doi: 10.1007/s12013-020-00909-2. Epub 2020 Apr 17. Cell Biochem Biophys. 2020. PMID: 32303898 Review.
-
[Racemic and optically active alpha-tocopherol analogues with a modified side chain: synthesis and biological activity].Bioorg Khim. 2007 Jul-Aug;33(4):387-404. doi: 10.1134/s1068162007040012. Bioorg Khim. 2007. PMID: 17886430 Review. Russian.
Cited by
-
The Effect of Antioxidants on Photoreactivity and Phototoxic Potential of RPE Melanolipofuscin Granules from Human Donors of Different Age.Antioxidants (Basel). 2020 Oct 26;9(11):1044. doi: 10.3390/antiox9111044. Antioxidants (Basel). 2020. PMID: 33114498 Free PMC article.
-
Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs.Open Vet J. 2023 Sep;13(9):1167-1174. doi: 10.5455/OVJ.2023.v13.i9.12. Epub 2023 Sep 30. Open Vet J. 2023. PMID: 37842116 Free PMC article.
-
Mechanisms enhancing the protective functions of macular xanthophylls in the retina during oxidative stress.Exp Eye Res. 2019 Jan;178:238-246. doi: 10.1016/j.exer.2018.06.012. Epub 2018 Jun 15. Exp Eye Res. 2019. PMID: 29908882 Free PMC article. Review.
-
Photodegradation of Lipofuscin in Suspension and in ARPE-19 Cells and the Similarity of Fluorescence of the Photodegradation Product with Oxidized Docosahexaenoate.Int J Mol Sci. 2022 Jan 15;23(2):922. doi: 10.3390/ijms23020922. Int J Mol Sci. 2022. PMID: 35055111 Free PMC article.
-
Nitroxide free radicals protect macular carotenoids against chemical destruction (bleaching) during lipid peroxidation.Free Radic Biol Med. 2016 Dec;101:446-454. doi: 10.1016/j.freeradbiomed.2016.11.012. Epub 2016 Nov 10. Free Radic Biol Med. 2016. PMID: 27840316 Free PMC article.
References
-
- Age-Related Eye Disease Study Research Group Pol J Environ StudA randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. - PMC - PubMed
-
- Aust O, Sies H, Stahl W, Polidori MC. Analysis of lipophilic antioxidants in human serum and tissues: tocopherols and carotenoids. J Chromatogr. A. 2001;936:83–93. - PubMed
-
- Beatty S, Chakravarthy U, Nolan JM, Muldrew KA, Woodside JV, Denny F, Stevenson MR. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120:600–606. - PubMed
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. - PubMed
-
- Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol. Vis. Sci. 2001;42:439–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

