Poly(A)-specific ribonuclease (PARN) mediates 3'-end maturation of the telomerase RNA component
- PMID: 26482878
- PMCID: PMC4791094
- DOI: 10.1038/ng.3423
Poly(A)-specific ribonuclease (PARN) mediates 3'-end maturation of the telomerase RNA component
Abstract
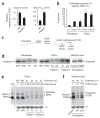
Mutations in the PARN gene (encoding poly(A)-specific ribonuclease) cause telomere diseases including familial idiopathic pulmonary fibrosis (IPF) and dyskeratosis congenita, but how PARN deficiency impairs telomere maintenance is unclear. Here, using somatic cells and induced pluripotent stem cells (iPSCs) from patients with dyskeratosis congenita with PARN mutations, we show that PARN is required for the 3'-end maturation of the telomerase RNA component (TERC). Patient-derived cells as well as immortalized cells in which PARN is disrupted show decreased levels of TERC. Deep sequencing of TERC RNA 3' termini shows that PARN is required for removal of post-transcriptionally acquired oligo(A) tails that target nuclear RNAs for degradation. Diminished TERC levels and the increased proportion of oligo(A) forms of TERC are normalized by restoring PARN, which is limiting for TERC maturation in cells. Our results demonstrate a new role for PARN in the biogenesis of TERC and provide a mechanism linking PARN mutations to telomere diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
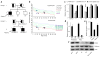




References
-
- Körner CG, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J Biol Chem. 1997;272:10448–10456. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

