Mitral valve disease--morphology and mechanisms
- PMID: 26483167
- PMCID: PMC4804623
- DOI: 10.1038/nrcardio.2015.161
Mitral valve disease--morphology and mechanisms
Abstract
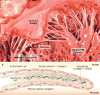
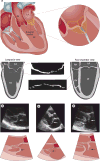
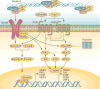
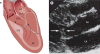
Mitral valve disease is a frequent cause of heart failure and death. Emerging evidence indicates that the mitral valve is not a passive structure, but--even in adult life--remains dynamic and accessible for treatment. This concept motivates efforts to reduce the clinical progression of mitral valve disease through early detection and modification of underlying mechanisms. Discoveries of genetic mutations causing mitral valve elongation and prolapse have revealed that growth factor signalling and cell migration pathways are regulated by structural molecules in ways that can be modified to limit progression from developmental defects to valve degeneration with clinical complications. Mitral valve enlargement can determine left ventricular outflow tract obstruction in hypertrophic cardiomyopathy, and might be stimulated by potentially modifiable biological valvular-ventricular interactions. Mitral valve plasticity also allows adaptive growth in response to ventricular remodelling. However, adverse cellular and mechanobiological processes create relative leaflet deficiency in the ischaemic setting, leading to mitral regurgitation with increased heart failure and mortality. Our approach, which bridges clinicians and basic scientists, enables the correlation of observed disease with cellular and molecular mechanisms, leading to the discovery of new opportunities for improving the natural history of mitral valve disease.
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Nkomo VT, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. - PubMed
-
- Enriquez-Sarano M, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. - PubMed
-
- Roberts R. Another chromosomal locus for mitral valve prolapse: close but no cigar. Circulation. 2005;112:1924–1926. - PubMed
-
- Williams TH, Jew JY. Is the mitral valve passive flap theory overstated? An active valve is hypothesized. Med Hypotheses. 2004;62:605–611. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL038176/HL/NHLBI NIH HHS/United States
- K24 HL067434/HL/NHLBI NIH HHS/United States
- R01 HL109506/HL/NHLBI NIH HHS/United States
- R01 HL127692/HL/NHLBI NIH HHS/United States
- P20 GM103444/GM/NIGMS NIH HHS/United States
- R01 HL128099/HL/NHLBI NIH HHS/United States
- R01 HL033756/HL/NHLBI NIH HHS/United States
- P30 GM103342/GM/NIGMS NIH HHS/United States
- R01 HL72265/HL/NHLBI NIH HHS/United States
- R01 HL072265/HL/NHLBI NIH HHS/United States
- R01 HL114805/HL/NHLBI NIH HHS/United States
- P20 RR021949/RR/NCRR NIH HHS/United States
- K23 HL116652/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

