Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase
- PMID: 26483191
- PMCID: PMC4664174
- DOI: 10.1093/hmg/ddv439
Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase
Abstract
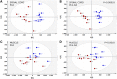
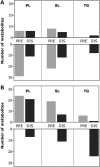
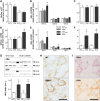
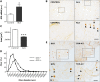
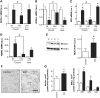
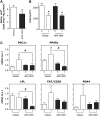
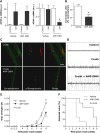
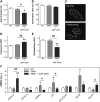
Amyotrophic lateral sclerosis (ALS) is a fatal adult-onset disease characterized by upper and lower motor neuron degeneration, muscle wasting and paralysis. Growing evidence suggests a link between changes in lipid metabolism and ALS. Here, we used UPLC/TOF-MS to survey the lipidome in SOD1(G86R) mice, a model of ALS. Significant changes in lipid expression were evident in spinal cord and skeletal muscle before overt neuropathology. In silico analysis also revealed appreciable changes in sphingolipids including ceramides and glucosylceramides (GlcCer). HPLC analysis showed increased amounts of GlcCer and downstream glycosphingolipids (GSLs) in SOD1(G86R) muscle compared with wild-type littermates. Glucosylceramide synthase (GCS), the enzyme responsible for GlcCer biosynthesis, was up-regulated in muscle of SOD1(G86R) mice and ALS patients, and in muscle of wild-type mice after surgically induced denervation. Conversely, inhibition of GCS in wild-type mice, following transient peripheral nerve injury, reversed the overexpression of genes in muscle involved in oxidative metabolism and delayed motor recovery. GCS inhibition in SOD1(G86R) mice also affected the expression of metabolic genes and induced a loss of muscle strength and morphological deterioration of the motor endplates. These findings suggest that GSLs may play a critical role in ALS muscle pathology and could lead to the identification of new therapeutic targets.
© The Author 2015. Published by Oxford University Press.
Figures
References
-
- Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. (2011) Amyotrophic lateral sclerosis. Lancet, 377, 942–955. - PubMed
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.X. et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 362, 59–62. - PubMed
-
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. et al. (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264, 1772–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

