Differential expression of endoplasmic reticulum stress-response proteins in different renal tubule subtypes of OVE26 diabetic mice
- PMID: 26483256
- PMCID: PMC4679738
- DOI: 10.1007/s12192-015-0648-2
Differential expression of endoplasmic reticulum stress-response proteins in different renal tubule subtypes of OVE26 diabetic mice
Abstract
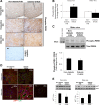
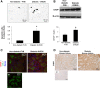
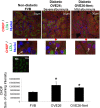
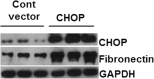
Regulation of the endoplasmic reticulum (ER) stress-response pathway during the course of diabetes specifically in renal tubules is unclear. Since tubule cell dysfunction is critical to progression of diabetic nephropathy, this study analyzed markers of ER stress response and ER chaperones at different stages of diabetes and in different renal tubule subtypes of OVE26 type-1 diabetic mice. ER stress-responseinduced chaperones GRP78, GRP94, and protein disulfide isomerase (PDI) were increased in isolated cortical tubules of older diabetic mice, while PDI was decreased in tubules of young diabetic mice. Immunofluorescence staining of kidneys from older mice showed GRP78 and PDI upregulation in all cortical tubule segments, with substantial induction of PDI in distal tubules. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) phosphorylation was increased in cortical tubules of young diabetic mice, with no differences between older diabetic and control mice. Expression of ER stress-induced PERK inhibitor p58IPK was decreased and then increased in all tubule subtypes of young and older mice, respectively. Knockdown of PERK by small interfering RNA (siRNA) increased fibronectin secretion in cultured proximal tubule cells. Tubules of older diabetic mice had significantly more apoptotic cells, and ER stress-induced proapoptotic transcription factor C/EBP homologous protein (CHOP) was increased in proximal and distal tubules of diabetic mice and diabetic humans. CHOP induction in OVE26 mice was not altered by severity of proteinuria. Overexpression of CHOP in cultured proximal tubule cells increased expression of fibronectin. These findings demonstrate differential ER stress-response signaling in tubule subtypes of diabetic mice and implicate a role for PERK and CHOP in tubule cell matrix protein production.
Figures


References
-
- Advani A, Gilbert RE, Thai K, Gow RM, Langham RG, Cox AJ, Connelly KA, Zhang Y, Herzenberg AM, Christensen PK, Pollock CA, Qi W, Tan SM, Parving HH, Kelly DJ. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–741. doi: 10.1681/ASN.2008020142. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

