Safety of oral dronabinol during opioid withdrawal in humans
- PMID: 26483357
- PMCID: PMC4663169
- DOI: 10.1016/j.drugalcdep.2015.09.031
Safety of oral dronabinol during opioid withdrawal in humans
Abstract
Background: Opioid dependence remains a significant public health problem worldwide with only three FDA-approved treatments, all targeting the mu-opioid receptor. Dronabinol, a cannabinoid (CB) 1 receptor agonist, is currently under investigation as a novel opioid withdrawal treatment. This study reports on safety outcomes of dronabinol among adults in opioid withdrawal.
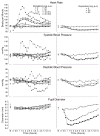
Methods: Twelve adults physically dependent on short-acting opioids participated in this 5-week within-subject, randomized, double blind, placebo-controlled inpatient study. Volunteers were maintained on oral oxycodone 30 mg qid. Double-blind placebo substitutions occurred for 21 h before each of 7 experimental sessions in order to produce opioid withdrawal. A single oral test dose was administered each session (placebo, oxycodone 30 and 60 mg, dronabinol 5, 10, 20, and 30 mg [decreased from 40 mg]). Heart rate, blood pressure, respiratory outcomes and pupil diameter were assessed repeatedly.
Results: Dronabinol 40 mg produced sustained sinus tachycardia accompanied by anxiety and panic necessitating dose reduction to 30 mg. Sinus tachycardia and anxiety also occurred in one volunteer after dronabinol 20mg. Compared to placebo, dronabinol 20 and 30 mg produced significant increases in heart rate beginning 1h after drug administration that lasted approximately 2h (p<0.05). Dronabinol 5 and 10mg produced placebo-like effects. Oxycodone produced prototypic mu-opioid agonist effects (e.g., miosis).
Conclusion: Dronabinol 20mg and higher increased heart rate among healthy adults at rest who were in a state of opioid withdrawal, raising concern about its safety. These results have important implications for future dosing strategies and may limit the utility of dronabinol as a treatment for opioid withdrawal.
Keywords: Dronabinol; Opioid dependence; Opioid withdrawal; Safety; Treatment.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Drs. Lofwall and Walsh have received honoraria from PCM Scientific for giving educational talks on opioid dependence andsalary support from Braeburn Pharmaceuticals for conducting clinical research at UK. Dr. Lofwall has consulted for CVS Caremark AND Orexo.
Mr. Nuzzo was a statistical consultant and project coordinator for the NIDA CTN Clinical Consulting Center and Johns Hopkins Behavioral Pharmacology Research Unit. Dr.Walsh has consulted for Sun Pharma, Camurus, World Meds, Durect, Novartis, Pfizer, Astra Zeneca, Cerecor, and Braeburn Pharmaceuticals. Dr. Elayi reports no conflicts.
Figures
Similar articles
-
Opioid withdrawal suppression efficacy of oral dronabinol in opioid dependent humans.Drug Alcohol Depend. 2016 Jul 1;164:143-150. doi: 10.1016/j.drugalcdep.2016.05.002. Epub 2016 May 10. Drug Alcohol Depend. 2016. PMID: 27234658 Free PMC article. Clinical Trial.
-
The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone.Drug Alcohol Depend. 2015 Sep 1;154:38-45. doi: 10.1016/j.drugalcdep.2015.05.013. Epub 2015 Jul 8. Drug Alcohol Depend. 2015. PMID: 26187456 Free PMC article. Clinical Trial.
-
Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans.Psychopharmacology (Berl). 2019 Nov;236(11):3341-3352. doi: 10.1007/s00213-019-05293-1. Epub 2019 Jun 15. Psychopharmacology (Berl). 2019. PMID: 31201479 Free PMC article. Clinical Trial.
-
Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence.Eur Neuropsychopharmacol. 2020 Jul;36:206-216. doi: 10.1016/j.euroneuro.2020.03.002. Epub 2020 Apr 6. Eur Neuropsychopharmacol. 2020. PMID: 32273144 Free PMC article. Review.
-
Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets.CNS Neurol Disord Drug Targets. 2013 Feb 1;12(1):112-25. doi: 10.2174/1871527311312010017. CNS Neurol Disord Drug Targets. 2013. PMID: 23244430 Review.
Cited by
-
What is the Current Knowledge About the Cardiovascular Risk for Users of Cannabis-Based Products? A Systematic Review.Curr Atheroscler Rep. 2017 Jun;19(6):26. doi: 10.1007/s11883-017-0663-0. Curr Atheroscler Rep. 2017. PMID: 28432636
-
Alleviation of opioid withdrawal by cannabis and delta-9-tetrahydrocannabinol: A systematic review of observational and experimental human studies.Drug Alcohol Depend. 2022 Dec 1;241:109702. doi: 10.1016/j.drugalcdep.2022.109702. Epub 2022 Nov 18. Drug Alcohol Depend. 2022. PMID: 36434879 Free PMC article.
-
Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach.Int J Environ Res Public Health. 2023 Feb 24;20(5):4087. doi: 10.3390/ijerph20054087. Int J Environ Res Public Health. 2023. PMID: 36901098 Free PMC article.
-
Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders.CNS Drugs. 2019 Oct;33(10):1001-1030. doi: 10.1007/s40263-019-00664-w. CNS Drugs. 2019. PMID: 31549358 Free PMC article. Review.
-
Non-Opioid Treatments for Opioid Use Disorder: Rationales and Data to Date.Drugs. 2020 Oct;80(15):1509-1524. doi: 10.1007/s40265-020-01373-1. Drugs. 2020. PMID: 32776315 Free PMC article. Review.
References
-
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther. 1979;25:440–446. - PubMed
-
- Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. - PubMed
-
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

