CaV3.2 T-type channels mediate Ca²⁺ entry during oocyte maturation and following fertilization
- PMID: 26483387
- PMCID: PMC4712821
- DOI: 10.1242/jcs.180026
CaV3.2 T-type channels mediate Ca²⁺ entry during oocyte maturation and following fertilization
Abstract
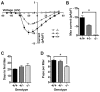
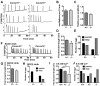
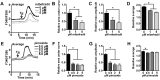
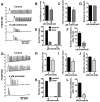
Initiation of mouse embryonic development depends upon a series of fertilization-induced rises in intracellular Ca(2+). Complete egg activation requires influx of extracellular Ca(2+); however, the channels that mediate this influx remain unknown. Here, we tested whether the α1 subunit of the T-type channel CaV3.2, encoded by Cacna1h, mediates Ca(2+) entry into oocytes. We show that mouse eggs express a robust voltage-activated Ca(2+) current that is completely absent in Cacna1h(-/-) eggs. Cacna1h(-/-) females have reduced litter sizes, and careful analysis of Ca(2+) oscillation patterns in Cacna1h(-/-) eggs following in vitro fertilization (IVF) revealed reductions in first transient length and oscillation persistence. Total and endoplasmic reticulum (ER) Ca(2+) stores were also reduced in Cacna1h(-/-) eggs. Pharmacological inhibition of CaV3.2 in wild-type CF-1 strain eggs using mibefradil or pimozide reduced Ca(2+) store accumulation during oocyte maturation and reduced Ca(2+) oscillation persistence, frequency and number following IVF. Overall, these data show that CaV3.2 T-type channels have prev8iously unrecognized roles in supporting the meiotic-maturation-associated increase in ER Ca(2+) stores and mediating Ca(2+) influx required for the activation of development.
Keywords: Ca2+; Egg activation; Fertilization; Meiosis; Oocyte; T-type channel.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization.Proc Natl Acad Sci U S A. 2018 Oct 30;115(44):E10370-E10378. doi: 10.1073/pnas.1810422115. Epub 2018 Oct 15. Proc Natl Acad Sci U S A. 2018. PMID: 30322909 Free PMC article.
-
Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs.Cell Calcium. 2017 Jul;65:63-72. doi: 10.1016/j.ceca.2017.02.004. Epub 2017 Feb 11. Cell Calcium. 2017. PMID: 28222911 Free PMC article.
-
Deletion of TRPV3 and CaV3.2 T-type channels in mice undermines fertility and Ca2+ homeostasis in oocytes and eggs.J Cell Sci. 2021 Jul 1;134(13):jcs257956. doi: 10.1242/jcs.257956. Epub 2021 Jul 12. J Cell Sci. 2021. PMID: 34313315 Free PMC article.
-
Role of store-operated calcium entry during meiotic progression and fertilization of mammalian oocytes.Int Rev Cell Mol Biol. 2012;295:291-328. doi: 10.1016/B978-0-12-394306-4.00014-9. Int Rev Cell Mol Biol. 2012. PMID: 22449493 Review.
-
Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs.Cell Calcium. 2013 Jan;53(1):63-7. doi: 10.1016/j.ceca.2012.11.010. Epub 2012 Dec 21. Cell Calcium. 2013. PMID: 23260016 Free PMC article. Review.
Cited by
-
Fertilization, Oocyte Activation, Calcium Release and Epigenetic Remodelling: Lessons From Cancer Models.Front Cell Dev Biol. 2022 Mar 4;10:781953. doi: 10.3389/fcell.2022.781953. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35309905 Free PMC article.
-
Exome sequencing to explore the possibility of predicting genetic susceptibility to the joint occurrence of polycystic ovary syndrome and Hashimoto's thyroiditis.Front Immunol. 2023 Jul 20;14:1193293. doi: 10.3389/fimmu.2023.1193293. eCollection 2023. Front Immunol. 2023. PMID: 37545519 Free PMC article.
-
Calcium oscillations in fertilized pig oocytes are associated with repetitive interactions between STIM1 and ORAI1.Biol Reprod. 2018 Apr 1;98(4):510-519. doi: 10.1093/biolre/ioy016. Biol Reprod. 2018. PMID: 29365044 Free PMC article.
-
Constitutive IP3R1-mediated Ca2+ release reduces Ca2+ store content and stimulates mitochondrial metabolism in mouse GV oocytes.J Cell Sci. 2019 Feb 12;132(3):jcs225441. doi: 10.1242/jcs.225441. J Cell Sci. 2019. PMID: 30659110 Free PMC article.
-
Divalent cation influx and calcium homeostasis in germinal vesicle mouse oocytes.Cell Calcium. 2020 May;87:102181. doi: 10.1016/j.ceca.2020.102181. Epub 2020 Feb 22. Cell Calcium. 2020. PMID: 32097818 Free PMC article.
References
-
- Arnoult C., Villaz M. and Florman H. M. (1998). Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol. Pharmacol. 53, 1104-1111. - PubMed
-
- Backs J., Stein P., Backs T., Duncan F. E., Grueter C. E., McAnally J., Qi X., Schultz R. M. and Olson E. N. (2010). The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc. Natl. Acad. Sci. USA 107, 81-86. 10.1073/pnas.0912658106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

